Compounding is an essential function of health system pharmacy practice, and achieving precision in compounding is vital to patient safety. As with most areas of pharmacy practice, a close eye for detail could be the difference between success and disaster. The Institute for Safe Medication Practices shed light on this when they reported on a case of a child who died when an oral product was compounded in error.1 The wrong active ingredient was incorporated into the compound, and a final check was either delayed or the ingredients were misidentified.
The American Society of Health-System Pharmacists (ASHP) has also addressed the importance of introducing barcode scanning in compounding.2 In their position statement, they support barcode scanning in compounding to enhance patient safety and quality of care by improving the accuracy of this core pharmacy function. They also detail how an audit trail can be created from barcode scanning. This trail can be used to track and trace a compounded product from pharmacy to patient.
The purpose of our project is to standardize compounding across our health system. By standardizing formulas for sterile and non-sterile compounds, and adding those formulas into the electronic health record (EHR), we are able to utilize ingredient barcode scanning to prevent tragic errors like the one highlighted here, and align with ASHP guidance. This process will not only prevent errors, it will also be used to link the ingredient lots and expiration dates to the patient record, creating the described audit trail, and making retrieval much less difficult in the event of a recall or compounding error.
The goals for this project are: standardize compounding processes across a multi-site health system with facilities ranging from a large medical center to critical access hospitals; build standardized compounding formulas into the EHR software; use barcode scanning during compounding to identify ingredients and document pharmacist verification; and reach 80% compliance with this process for all facilities within the health system.
Our health system uses Epic® as the EHR. It has activities called Compounding and Repackaging (CNR) and Dispense Prep, which can be used to accomplish the goals of this project. These activities require configuration, but are included in our software system at no additional cost. They can be incorporated without the time or expense required to procure an outside software vendor. We convened a team of pharmacists and technicians from across our health system that worked together to compile a list of commonly-used compounds. We standardized the compounding procedure for each formula and assigned common Beyond Use Dates (BUDs), using primary literature references when possible, or referencing USP <797> and <795> when not available.
Once the formulas and BUDs were finalized, we consulted with our IT pharmacy support team to build the compounds in the EHR, ensuring procedures and functionality could adapt to the needs of our facilities of varying scope and size. Both CNR and Dispense Prep allow for flexibility in vial size used during compounding—one facility could use a 1g vial of vancomycin, for instance, while another could use a 10g vial.
During compounding, pharmacy staff accesses the CNR and Dispense Prep activities within the Epic® Willow module. Dispense Prep is intended for use in patient-specific compounding, and will accordingly link the ingredient lots and expirations to the final dispensing on the patient’s EHR. CNR is intended for batch compounding, and will link component lot number and expiration information to a patient when a nurse scans the medication at bedside.
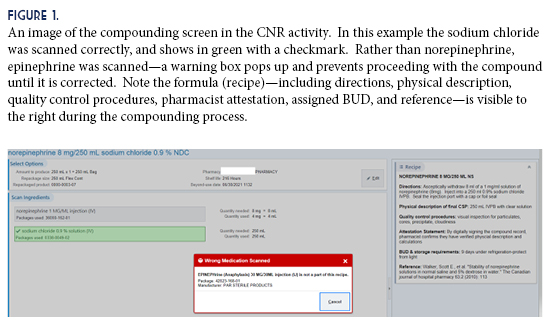
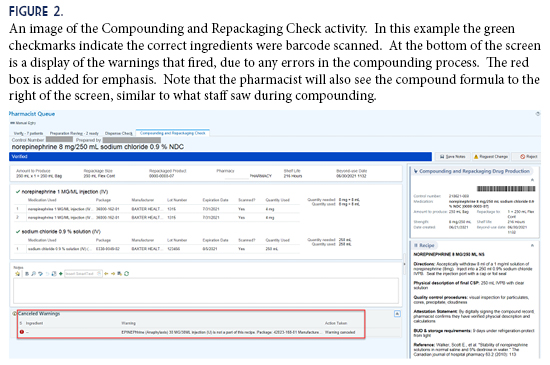
To produce a batch compound using CNR, pharmacy staff selects the desired CNR record to display the formula and ingredients (Figure 1). If an ingredient is incorrectly scanned, a warning message will display, and cannot be bypassed until it is rectified. Once the compound is completed, the verifying pharmacist uses another activity called Compounding and Repackaging Check. Here the label barcode is scanned again and a summary screen will display the ingredients used, as well as any errors that occurred (Figure 2). When the pharmacist is satisfied with the compounding accuracy, they can verify and approve the compound. If errors cannot be explained during the review, the pharmacist can also reject the compound, in which case it would route back to be compounded again. The pharmacist uses a similar activity called Dispense Check to verify patient-specific doses using Dispense Prep.
Experience with Outcomes of the Program
We almost immediately realized how complex standardizing compounding would be. In addition to internal capabilities, Epic® also receives information from Medi-Span®, our medication data vendor. The way our health system uses Medi-Span®’s classifications of diluents can limit their interchangeability. To continue the example used earlier, we can use different vial sizes of vancomycin, but we cannot switch between sterile water for injection vials and bags—vials are categorized in Medi-Span® as injections (IJ SOLN), and bags are listed as infusions (IV SOLN). This meant we needed to further standardize, not only to formula and BUD, but also to which presentation of diluent was used throughout the health system.
Once Dispense Prep and CNR were rolled out across the system, they were widely accepted. Electronically capturing our compounding efforts meant we no longer had to keep often lengthy paper logs to comply with regulatory requirements. It also increased our productivity and reproducibility—the need to hand-write documentation was eliminated, and the ability to electronically pull up the record if needed prevented a laborious search, both saving time. In the first six months of Fiscal Year 2021, system-wide compliance with Dispense Prep has reached 64.6%, with a goal to reach 80% compliance by Fiscal Year-end.
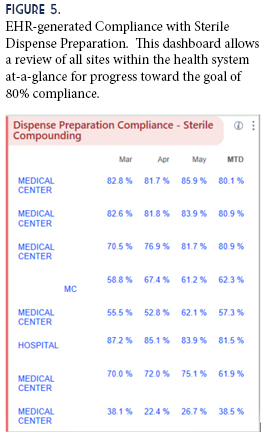
An unexpected benefit from the project was an added layer of protection against administering expired compounds. Since the formulas and BUDs are built into the EHR, the software can track expiration dates. If a compound is expired, nursing staff is alerted via a pop-up message during administration (Figure 3). This warning can be overridden, but it does add another tier of safety that may prevent administration errors potentially leading to patient harm. Overriding a warning generates trackable data that can be pulled and analyzed for trends or opportunities for additional training.
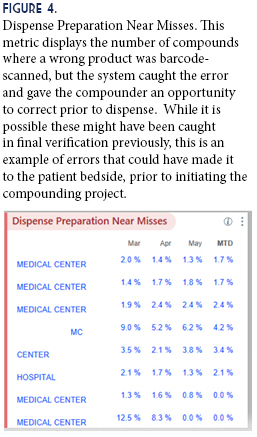
Studies have shown that barcode scanning can result in a relative reduction in the incidence of errors by 93-96%.3 This is particularly impactful in compounding, where errors are difficult to detect once they leave the pharmacy. Prior to our project, verification of compounding was an entirely human process. Implementing barcode scanning and standardized compounding formulas allow us to be more confident in our ability to identify compounding errors before they reach the patient. Additionally, our IT pharmacy support team was able to use captured data to generate a Near Misses dashboard (Figure 4). This dashboard allows us to review software-discovered issues in compounding, to identify trends and reinforce training where needed.
Another benefit of the program is the pass-through of drug NDC numbers, from wholesaler ordering to patient dispensing. A mismatch of NDC numbers, where one is dispensed and another is documented or billed, is the definition of misbranding. While this is prohibited in the Food, Drug, and Cosmetic Act, accuracy of billed NDC numbers is also especially important for facilities that participate in the HRSA 340B Drug Pricing Program. Where applicable, facilities are granted accumulations of drugs at a discounted rate, based on use in low-income or at-risk patient populations. Verifying NDC pass-through ensures compliance with the HRSA program, improves regulatory compliance, and maintains health system cost-savings from that program. The compounding project enables our health system to track and trace NDC pass-through, which validates our compliance with the 340B Drug Pricing Program requirements.
Discussion of Innovative Aspects of Program and
Achievement of Goals
This project proved to be an innovative and far-reaching application at our health system. Not only did it meet the initial goal of identifying and preventing the dispensing of compounds made in error, it also improved productivity of pharmacy staff by eliminating paper documentation, aided in validation of our 340B program, and added another layer of awareness to nursing staff to prevent accidental administration of expired compounds.
One of the major undertakings of this project was to develop standardized compounding formulas, and have them built within the EHR. This ensured that all sites were following the same procedure, resulting in a consistent patient and healthcare team experience, no matter where that person was within the health system. Previously we relied on paper formulas that could be easily lost or become obsolete, or could be misinterpreted when transcribed from one location to another. Now our EHR is the source of truth, eliminating many of those duplicative and time-consuming risks.
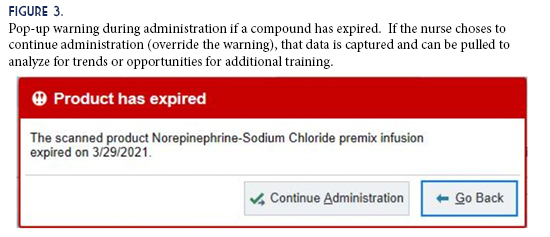
Another innovative aspect was the development of metrics to allow us to consistently measure our compliance with the Epic® activities. Previously we did not have an easily-measurable metric to validate compounding compliance. Now we can pull compliance data from our EHR, comparing all facilities within the health system for Dispense Prep (Figure 5). This filled a gap in Quality, Safety, and Regulatory oversight, and satisfies many of the questions in this area that are frequently raised during inspections or surveys. Development of the metrics led to a new standard for compliance—the goal of 80% use of Dispense Prep by Fiscal Year-end. This goal has yet to be reached, and is ongoing.
Conclusion
Utilizing software-aided compounding, through barcode scanning and documentation, has been a great success for our health system. Standardized formulas, built in the EHR, ensure that all facilities are consistently producing compounds with the same process. Adopting BUDs that flow through the EHR provides added protection against administration of expired compounds. Ingredient barcode scanning adds greater oversight, where a pharmacist can see at-a glance if the compound was prepared correctly, and has led to an increased confidence in the accuracy of our compounded products. The pass-through of drug NDC numbers to patient administration provides additional benefits, preventing misbranding and preserving beneficial purchasing through government programs. Finally, being able to utilize activities that already exist in our EHR allowed us to make these gains in safety and accuracy without a large capital investment.
Our experience has shown that, while there is considerable investment in time to develop a program utilizing software aided compounding, the benefits are numerous, to both the health system and the patients we serve. Some of the positive impacts were not even apparent until after we started the project. We would encourage any health system to investigate the possibilities within their own EHR to do the same.
References
- Institute of Safe Medication Practices. Death Due to Pharmacy Compounding Error Reinforces Need for Safety Focus. June 15, 2017. Available at Death Due to Pharmacy Compounding Error Reinforces Need for Safety Focus | Institute For Safe Medication Practices (ismp.org) Accessed June 21, 2021.
- American Society of Health System Pharmacists. ASHP statement on barcode verification during inventory, preparation, and dispensing of medications. Am J Health-Syst Pharm. 2011; 68-442-5
- Poon EG, Cina JL, Churchill W, Patel N, et al. Medication dispensing errors and potential adverse drug events before and after implementing bar code technology in the pharmacy. Ann Internal Med. 2006 Sep 19; 145(6): 426-34