Print Entire Issue
Columns
President's Message
Directly Speaking
Crank's Corner
Board of Pharmacy Update
Government Affairs Report
Professional Affairs
Organizational Affairs
Hi-Tech
ICHPeople
ICHP Leadership Spotlight
ICHP Leadership Spotlight
ICHP Champions Update
There's Still Time to Step Up!
We Need Reasons to Celebrate This Year!
Educational Affairs
Educational Affairs
Educational Affairs
Educational Affairs
Educational Affairs
Features
Opioid Task Force - CPE Opportunity!
Another Perspective
2020 ICHP Annual Meeting
College Connection
Midwestern University Chicago College of Pharmacy
Roosevelt University College of Pharmacy
Rosalind Franklin University College of Pharmacy
Southern Illinois University Edwardsville School of Pharmacy
More
Upcoming Events
Welcome New Members!
Pharmacy Action Fund Contributors
ICHP Board of Directors 2020 - 2021

Illinois Council of Health-System Pharmacists
4055 North Perryville Road
Loves Park, IL 61111-8653
Phone: (815) 227-9292
Fax: (815) 227-9294
ichpnet.org
KeePosted
Official News journal of the Illinois Council of Health-System Pharmacists
EDITOR
Jennifer Phillips
ASSISTANT EDITOR
Milena Murray
MANAGING EDITOR
Scott Meyers
ASSISTANT MANAGING EDITOR
Trish Wegner
DESIGN EDITOR
Melissa Dyrdahl
ICHP Staff
EXECUTIVE VICE PRESIDENT
Scott Meyers
VICE PRESIDENT - PROFESSIONAL SERVICES
Trish Wegner
DIRECTOR OF OPERATIONS
Maggie Allen
INFORMATION SPECIALIST
Heidi Sunday
CUSTOMER SERVICE AND
PHARMACY TECH TOPICS™ SPECIALIST
Jo Ann Haley
ACCOUNTANT
Jan Mark
COMMUNICATIONS MANAGER
Melissa Dyrdahl
LEGISLATIVE CONSULTANTS
Liz Brown Reeves
Mitch Schaben
ICHP's Mission Statement
Advancing Excellence in
Pharmacy
ICHP's Vision Statement
ICHP dedicates itself to
achieving a vision of pharmacy practice where:
·
Pharmacists are
universally recognized as health care professionals and essential providers of
health care services.
·
Pharmacists use their
medication expertise and leadership skills to optimize the medication use
process and patient outcomes.
·
Pharmacy technicians are
trained and PTCB certified to manage the medication distribution process.
ICHP's Goal Statements
·
Raising awareness of the
critical role pharmacists fulfill in optimizing medication therapy and ensuring
medication safety in team-based, patient-centered care.
·
Providing high quality
educational services through innovative continuing pharmacy education and
training programs, and sharing evidence-based best practices.
·
Developing and nurturing
leaders through mentorship, skill development programs, and leadership
opportunities.
·
Working with national
and state legislators and policymakers to create or revise legislation and
regulation critical to pharmacy practice and quality patient care.
·
Urging pharmacy
technician employers to require successful completion of an accredited pharmacy
technician training program and PTCB certification of all pharmacy technicians.
Approved by the ICHP
Board of Directors May 30, 2018.
KeePosted Vision
As an integral publication of the Illinois Council of Health-System
Pharmacists, the KeePosted newsjournal will reflect its
mission and goals. In conjunction with those goals, KeePosted will
provide timely information that meets the changing professional and personal
needs of Illinois pharmacists and technicians, and maintain high publication
standards.
KeePosted is an official publication of, and is copyrighted by, the
Illinois Council of Health-System Pharmacists (ICHP). KeePosted is
published 4 times a year. ICHP members received KeePosted as
a member benefit. All articles published herein represent the opinions of the
authors and do not reflect the policy of the ICHP or the authors’ institutions
unless specified. Advertising inquiries can be directed to ICHP office at the
address listed above. Image disclaimer: The image used in the Pharmacy Tech
Topics™ advertisement is the property of © 2017 Thinkstock, a division of Getty
Images. Some images are property of © 2020 Adobe Stock.
Copyright © 2020, Illinois Council of Health-System Pharmacists. All rights
reserved.
Columns
 President's Message
President's Message
Black Lives Matter On Our Team
by Carrie Vogler, PharmD, BCPS, Clinical Associate Professor - SIUE School of Pharmacy
Greetings ICHP!
We are in a time of change; change can feel disruptive and difficult but is crucial to our lives and our development. With everything that is happening these days and so many uncertainties, sometimes I just need to take a step back. The “new normal” continues to shape itself and I remain hopeful for what that will look like. We are working through a pandemic, dealing with economic disparities, and facing racial inequality and social injustices all at the same time. I will argue that our patients and our colleagues need one another more than ever to unite and overcome these challenges.
I am proud of the continued efforts made by my colleague Dr. Lakesha Butler at SIUE School of Pharmacy and President of the National Pharmaceutical Association to help to unite 13 pharmacy organizations to make a stand against racial injustice for Black Americans.1 In June, ASHP announced the creation of a Task Force on Racial Diversity, Equity, and Inclusion to advise ASHP on specific, actionable steps to further address and take inventory of matters of racial diversity, equity, and inclusion. These are encouraging steps that the profession is taking to address the disparities that currently exist in our country. I think about the number of patients I have served who have been at a disadvantage in terms of opportunities to receive the best health care possible. I hope the current conversations, education, and actions being taken will bring these inequalities to our immediate attention and lead to vital changes to overcome them. It is also my hope that the current under-representation of Black pharmacists and relative lack of diversity in our profession can be improved as the barriers that have historically prevented inclusion within the profession in pharmacy are systematically dismantled.
We are all hurting right now in some way or another. The conversations we need to have are sometimes difficult. Navigating a conversation with my eight-year-old daughter, trying to explain what happened to George Floyd and the subsequent activism was not easy, but needed to be addressed. In school, she had learned about Dr. Martin Luther King, Jr. and the stories that shaped our country’s history. I felt crushed to tell her that the stories she had read and the things that Dr. Martin Luther King, Jr. stood for were still happening TODAY. I am working through my disappointment in myself for the countless number of times I have seen events showing injustice in the news and within my community and the eventual numbness I began to feel toward this “routine” reporting. I want to be a part of the solution so that no person has to continue to face these injustices.
As difficult as these times are, I remind myself to be thankful for the lessons they teach us and for the opportunities provided for me to grow as a person. It keeps me grounded and helps me put things in perspective. It is more important than ever to spend time reaching out to our teammates to check in on them, to ask about their day. I appreciate all of the people who take time to show they care and are actively serving our patients and communities. We need you as our frontline heroes and our advocates.
We need to make an effort to listen, to connect, and to educate ourselves. I recognize that there are many stories and circumstances that I am unaware of and I need to make an effort to learn more about inequality, recognize my unconscious bias, and keep the conversation going.
I encourage you to think about what you can do to help bring equality to Our Team and within the efforts of ICHP. Let’s be part of the solution and continue to learn and grow together. Spread kindness, hope, and compassion to everyone. Ask yourself, what can I change? ■
- ASHP Joins National Pharmacy Organizations to Stand Against Racism Press Release 6/5/2020. Access 6/16/20 at: www.ashp.org/News/2020/06/05/ASHP-Joins-National-Pharmacy-Organizations-to-Stand-Against-Racism
 Directly Speaking
Directly Speaking
Personal Freedom or Personal Responsibility
by Scott A. Meyers, Executive Vice President
The United States was founded on the principles of personal freedom, life, liberty, and the pursuit of happiness. It says so in the Declaration of Independence. But continued life, liberty, and the pursuit of happiness come at the cost of personal responsibility for all the citizens of this great nation.
Unfortunately, it seems that we have seen a steady erosion of the latter as more and more citizens seek the former. Perhaps the most recent example of this is the anti-mask movement in the U.S. The COVID-19 pandemic has created the need to wear masks out in public and for most of you, at work daily, and yet many of our neighbors have taken a stand, foolish as it is, to rail against the masks as a form of government control and loss of freedom.
Seems all too similar to the rebellion of anti-vaxxers we have seen for many years. “The government can’t tell me what to do!” “The government can’t make decisions for my children!” “There’s no proof these vaccines work!” All, this based on one doctored study and a strong mistrust of science!
On another front, fortunately, we are now seeing many citizens stepping up to take personal responsibility with regard to racism in the United States. So many times in this country, white people have witnessed the injustices to Black, Latino and other minorities and have done nothing. That seems to be changing but will the numbers be enough? Are those people stepping up going to outnumber those still practicing racism? Will they step out far enough to call out those perpetuating these injustices?
It seems to me that we, as a country, and as individuals, have lost the drive or determination to do the right thing. That’s really what personal responsibility is. Not just personally acting responsible by wearing a mask or being vaccinated, but stepping out of our comfort zone and doing the right thing for others.
Yes, it gets uncomfortable when you do the right thing sometimes. Sometimes it even gets dangerous. But it seems like the number of people willing to risk comfort and even themselves for others continues to decline and we need to turn that around. We need to speak out, band together, or reach out to those in authority to make the changes that need to be made. An example that comes to my mind is when George Floyd was being held down with a knee on his neck. Why didn’t at least one bystander call 911 and ask for more help and tell the dispatcher what was happening as it occurred? If someone did, we haven’t heard about it. Yes, thank goodness, someone made the video but there were others watching who could have done more. I know why they didn’t argue or try to remove the police officers from Mr. Floyd’s neck. That would have been disastrous, but calling for more police who might be more restrained could have saved Mr. Floyd’s life.
Maybe it’s time to push ourselves just a little more into the uncomfortable zone when we see something that just isn’t right? We need to band together if necessary to let someone know they are wrong in what they are doing. We need to reach out to find others who will take a risk with us for someone else to make sure that person has the same chance at life and liberty. If we all take a little extra personal responsibility, maybe everyone will be able to enjoy their personal freedom. ■
Crank's Corner
I'd Like To Get To Know You
by Christopher W. Crank, Incoming Executive Vice President
I am honored to have been chosen to be the next Executive Vice President of the Illinois Council of Health-System Pharmacists. I look forward to working with the ICHP membership to advance excellence in pharmacy. I am an Illinoisan born and raised. I grew up in a small town in north central Illinois called Washburn. (Yes, Chicago members, the Peoria area is north central Illinois; Springfield is central Illinois.) While in junior high and high school I was fortunate to have excellent science teachers. Eric Rittenhouse and Brad Stork were instrumental in my education. They were great at teaching scientific theory. Most importantly, they excelled at teaching practical application of science and made learning science engaging and fun. They opened my eyes to opportunities that existed in the field of science. I truly cannot thank them enough.
My interests in science and pharmacy were a perfect combination. I wanted to work in a science field while being able to help people. I attended the University of Iowa College of Pharmacy where I discovered that I wanted to be a clinical pharmacist involved in direct patient care. I completed an Internal Medicine residency with the St. Louis College of Pharmacy with a practice site of the John Cochran Veterans Hospital. After completing my residency, I stayed on with the St. Louis College of Pharmacy as an Assistant Professor. In 2003, I had the opportunity to join the staff of Rush University Medical Center. I developed my clinical skills, gained leadership experience, pursued scholarly activity, published research, and improved my ability to educate others. While at Rush, I became interested in gaining a better understanding of the healthcare system as a whole. For this reason, I obtained a Master’s of Science degree in Health System Management in 2009. In 2014, I became the Director of Pharmacy Services at Rush Copley Medical Center.
All of the information above describes my background, but not who I am. I would describe my leadership style as diplomatic. I work hard to keep individuals working together toward a common goal. In addition, I am a very social person who likes to get to know the people I am working with. If I was asked to describe myself in a couple of words, I would use the following words: gregarious and determined. If I asked my wife, past students and residents, and colleagues they would add talkative to the list.
My first experience with ICHP was as a presenter at an ICHP meeting. I remember being impressed with the educational offerings and the opportunities to network. I joined ICHP shortly thereafter in 2006. Since that time, I have volunteered with ICHP in many different roles, including as a member of the Government Affairs Division, Director of the Government Affairs Division, a member of the PAC Board of Trustees, and as ICHP Treasurer. Throughout my time with ICHP, I have seen the critical role the organization plays in advocacy, education, and networking opportunities. I have grown professionally because of my involvement with the organization.
When the Executive Vice President (EVP) position opened up, I knew it was an excellent opportunity to make a difference. During the COVID-19 pandemic, I have frequently found myself wondering why there isn’t more recognition of pharmacists and technicians. Why do U.S. senators not know what pharmacists and technicians do for patient care? Why doesn’t the healthcare system utilize pharmacists and technicians to our full potential? I pursued the EVP position because I want to promote the excellent patient care that we provide and work to expand our roles.
One challenge that we are facing in health-system pharmacy that I would like to address is the increasing trend of insurance companies dictating where infusion therapies may be delivered. In an effort to reduce the cost of the infusions, patients are not allowed to use hospital-based infusion centers associated with their providers. While most of us would agree that decreasing cost of care is a reasonable goal, we would not agree that safety should be compromised. When examining cost of the infusions, it is critical to examine what safety measures are in place at the infusion centers. Is the product prepared in a USP 797- and 800-compliant clean room? Is there a pharmacist to double check for high risk drugs? Are smart pumps used to infuse the products? It is my opinion that we need to advocate to key stakeholders that hospital-based infusion centers provide some patient safety advantages that other sites of care do not. It is also important that hospital-based infusion centers adjust their charges to be more in line with true cost of delivering the care.
If there is one thing that I would ask each of you as members of ICHP to do, it would be to get involved. I believe that each member of the organization has something to contribute. One key area to get involved in is advocacy. The Illinois Pharmacy Practice Act is up for renewal in 2023, and the Collaborative Pharmaceutical Task Force continues to meet to address key aspects of pharmacy practice. It is critical that we meet with our legislators so they get a better understanding of what we do and to convey our concerns about proposed legislation. We cannot rely on others to step in for us. It is the responsibility of each pharmacist and technician to advocate for our profession. If we don’t fight for pharmacy, others will not do it for us.
In the end, I hope to serve the members of ICHP well. I will work hard to advocate for positive change for our profession. I will build upon my professional network to help pharmacists and technicians make needed connections. I will ensure ICHP continues to provide excellent educational material. I will strive to keep ICHP strong financially so it may continue to support our members. I look forward to working with all of you. ■
Board of Pharmacy Update
Highlights of the May 2020 Meeting
by Scott A. Meyers, Executive Vice President
The May 12th Board of Pharmacy Meeting was held via conference call due to the COVID-19 pandemic. These are the highlights of that meeting.
Announcements – A brief description of the revised NABP Annual Meeting was provided by Board Chair, Denise Scarpelli. The meeting has been reduced to a one-day policy meeting with one delegate representing each member state. Ms. Scarpelli will represent Illinois.
Department update - Staff member and Board General Counsel, Munaza Aman provided a review of actions taken by the Department in relation to the COVID-19 pandemic. Notices of each action appear on the Department’s website.
Legislative Update - Garth Reynolds presented the Legislative Update to the Board and reported that the General Assembly has been suspended and will most likely meet later this month. Garth requested more action from the Department on relaxing regulations for pharmacists to help them better serve the citizens of Illinois during the pandemic.
Public Comments - During the public comment session of the meeting there was significant discussion regarding licensure of new graduates. With the limited number of seats available at the testing centers and the very slow response of the contractor for the State of Illinois, there is serious concern for new residents and other new hires being able to work unsupervised.
Next Meeting – The July 14th Board of Pharmacy meeting will be reported on in the November 2020 KeePosted. These meetings are open to the public and pharmacists, pharmacy technicians and pharmacy students are encouraged to attend. ■
Government Affairs Report
We Have a Budget and a Slow Moving Task Force
by Scott A. Meyers, Executive Vice President
Since the last issue of the KeePosted, published in May of this year, the General Assembly did reconvene for a much shorter than usual Spring Session. They managed to pass a budget in the 3-4 day shortened session along with a few other bills -many related to the COVID-19 pandemic and a few more that prevented several sunsets from occurring. Fortunately for pharmacy, no major damage was done, but that really means that we can expect more than the usual headaches from this fall’s veto session.
The Task Force met via conference call on Monday, May 11th and again on Tuesday, June 16th. During the May call, the Task Force voted to recommend new Rules related to Section 15.1 of the Act. That is the new section on Pharmacy Working Conditions. The proposed rule would exempt pharmacy residents from the 12-hour shift length limit if they were participating in a nationally accredited residency program whose program rules met the national duty hour standards.
The Task Force also discussed the use of NABP’s CPE Monitor by the State of Illinois to monitor pharmacist and certified technician compliance with the Act’s continuing education requirements. No final vote was taken.
At the June 16th Meeting of the Task Force, there was discussion on clarifying additional requirements of Section 15.1 of the Act pertaining to taking meal breaks and their documentation. There was a push to exempt pharmacists who do not have product verification responsibilities during their work shifts. This proposal was met with some concern and was referred to the next meeting to clarify the definition of those who could be exempted from the requirement to take and to document meal and rest breaks.
The notes from the July Task Force will be available in the next KeePosted. You can also monitor the Department of Financial and Professional Regulation website at
www.idfpr.gov.
Finally, ICHP continues to provide member assistance with the COVID-19 pandemic and the ICHP office staff continue to update the COVID-19 Resource page on the ICHP website. The page includes a variety of documents including:
- CDC COVID-19 website
- ASHP COVID-19 resource page
- Multiple documents from State of Illinois Agencies and IPhA
- Updates from USP
ICHP staff continue to post new resources as they are available and continue to conduct medication shortage surveys with the results provided to the Illinois Department of Public Health, the Illinois Emergency Management Agency and the U.S. Food and Drug Administration. ■
Professional Affairs
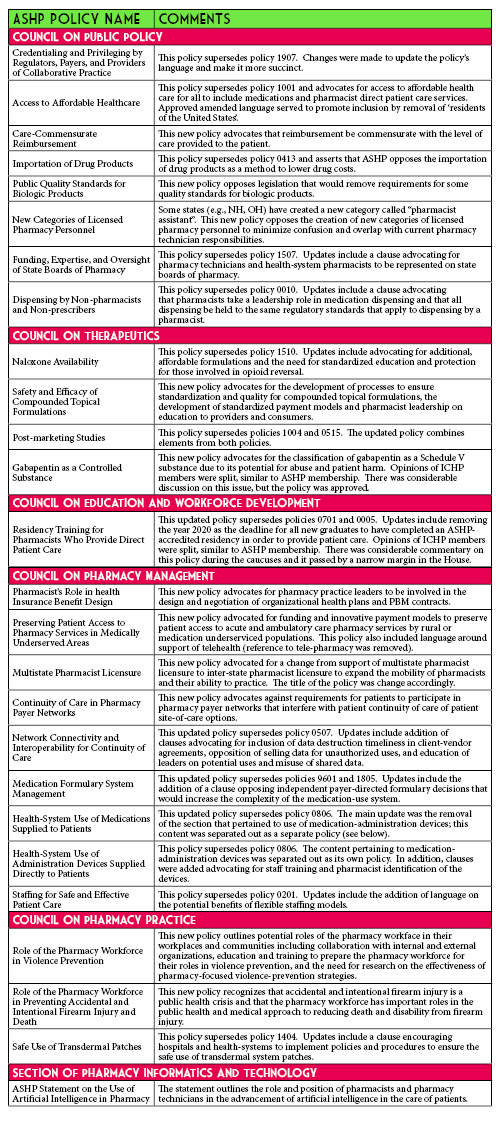
2020 ASHP House of Delegates Update
by Jen Phillips, PharmD, BCPS, FCCP, FASHP; Charlene Hope, PharmdD, MS, CPPS; Andrew Donnelly, BS, PharmD, MBA; Bernice Man, PharmD, BCPS; Chris Crank, PharmD, MS, BCPS AQ ID
In June 2020, the ASHP House of Delegates met virtually for the first time ever due to the cancellation of the live ASHP Summer Meeting. The House debated 26 policies. All policies were approved, with 14 being amended or edited by the house. This year’s Illinois delegates were: Christopher Crank, Andy Donnelly, Charlene Hope, Bernice Man, and Jennifer Phillips. While the full version of the policies under consideration as well as ASHP member’s comments can be found on the ASHP connect website, the following table gives a brief overview of the approved policies.
Thanks to all the ICHP members who provided feedback on the policies before the meeting. The delegates took your recommendations into consideration when representing the membership.
We look forward to the deliberations of the next House of Delegates Meeting, scheduled for June 2021 in Long Beach, CA! Hope to see you there! ■
Organizational Affairs
ICHP Organizational Affairs Knowledge
DID YOU KNOW?
Paul Abramowitz, the Chief Executive Officer of ASHP, used to live in Illinois. During his term as President-Elect of ICHP, he relocated to Minnesota and did not serve as President of ICHP due to living and practicing out of state.
ICHP ORGANIZATIONAL AFFAIRS KNOWLEDGE
ICHP members who wish to hold office must be either a resident of or have current employment within the state of Illinois. ■
Hi-Tech
All The Ways You Can Be Involved
by Becky Ohrmund, CPhT, Pharmacy Technician Specialist; Northwestern Memorial Hospital; ICHP Technician Representative-elect
Hi, ICHP Technicians!!!
Do you know all of the things that techs can do within ICHP? Below is just a short list of some of the Divisions that you can belong to as well as some of the Committees that you can join:
- Educational Affairs Division
- Government Affairs Division
- Marketing Affairs Division
- Organizational Affairs Division
- Professional Affairs Division
- Technology Committee
- KeePosted Committee
- Pharmacy Tech Topics™ Steering Committee
- Annual Meeting and Spring Meeting Planning Committees
- Technician Network
If you are looking for a short-term opportunity, consider one of the following:
- Annual and Spring Meeting Speakers
- Annual and Spring Meeting Moderators
- Annual and Spring Meeting Registration Desk Volunteers
- Spring Meeting Poster Presenter
- Spring Meeting Poster Abstract Reviewer
- Spring Meeting Poster Judge
- Legislative Day Participant
- Legislative Day Group Leader
- Authors for the KeePosted or Pharmacy Tech Topics™
- Editors and Peer Reviewers for articles in the KeePosted or Pharmacy Tech Topics™
I first got involved with ICHP when I volunteered for the Spring planning committee. I have been on all of the planning committees since that first one and I love being able to help plan out the technician sessions at the meetings. This allows me to pick topics that are being presented from Technicians by Technicians. I have also been to Legislative Day, served as a Moderator for meetings, and completed peer reviews. If any of the above opportunities jump out at you as something you want to do, please contact me or go to the ICHP website and click on Volunteer sign up under the About Us tab. A member of the Division or Committee that you would like to join will contact you with more information. If you would like to be a part of the planning committee, please reach out to myself or David Martin (Director of Educational Affairs).
Remember that how far you want to go starts with you! ■
ICHPeople
Congratulations Dr. Marianne Pop!
Advisor of the Semester!
Dr. Marianne Pop was recognized by the Rockford PhLAMES student advisory group as the Spring 2020 Advisor of the Semester.
Dr. Pop's willingness to go above and beyond for mentoring, being readily available and willing to help at all times were noted by the group.
Congratulations Marianne!
Dr. Pop is ICHP's NPN Director-elect.
ICHP Leadership Spotlight
Meet Ashlie Kallal, PharmD, CLSSBB
What is your leadership position within ICHP?
I am the Sangamiss Chapter President-Elect. My primary responsibility in this role is to plan the monthly CPE meetings for our members.
Describe your practice site.
I am the Supervisor of Medication Safety at Memorial Medical Center. As the Medication Safety Officer, I am responsible for the quality and safety of the medication use process. I also serve as a PGY-1 preceptor for the Medication Safety learning experience and am responsible for our Investigational Drug Service.
How did you know that pharmacy was for you?
Attending pharmacy school at Butler University was a clear career choice for me. I had always been interested in the medical field with special interest in science and math. My sister attended Butler University for pharmacy, and I fell in love with Butler during the many weekends I spent visiting her over the years. I love what I do and know that pharmacy was the right choice for me.
What are some challenges that you face in your practice?
A current challenge - and a medication safety focus – involves the safe use of opioids. This issue touches almost every patient we encounter. We have worked to identify high-risk patient populations and to implement process improvements to reduce the risk of opioid associated ADEs. Our current focus is safe opioid use in patients with renal insufficiency. We recently implemented additional electronic alerts to identify these at-risk patients at the time of order entry. We will continue to monitor prescribing habits to evaluate if these alerts have made a positive impact on opioid prescribing in this patient population.
What makes ICHP great?
ICHP offers many great leadership opportunities. There are so many different ways you can become more involved, and you can do so at a level that you are comfortable with, as your time allows. Not only will you benefit from these experiences, you are also positively impacting the pharmacy profession and your pharmacy peers.
Tell us about when your first experience with ICHP.
I initially joined ICHP to participate in the monthly Sangamiss CPE meetings. I am able to network with pharmacy peers while earning CPE and enjoying dinner and drinks! I now know more about all that ICHP has to offer, but these monthly meetings remain a personal favorite.
Do you have any advice for students?
I think it is important that as a pharmacist you become a self-learner. Pharmacy is constantly changing and evolving, and we must continue to learn and grow our skills and knowledge.
What special interests or hobbies do you enjoy outside of work?
I am a mom to three boys and spending time as a family is what I enjoy most of all. We enjoy traveling and camping, and I love to cook. I am always trying new recipes and picking up tips and tricks from The Food Network.
Where is your favorite place to vacation?
I love to travel and cannot narrow it down to a single favorite. We did recently take an amazing trip out west. We drove over 5,000 miles through ten states and visited five national parks. Yellowstone was the highlight of this trip for me. Our next big adventure to Alaska was planned for this summer but has been rescheduled due to the COVID-19 pandemic. ■
ICHP Leadership Spotlight
Meet Becky Ohrmund, CPhT
What is your leadership position within ICHP?
I am the Technician Representative-Elect.
Where is your practice site?
I am a Pharmacy Technician Specialist at Northwestern Memorial Hospital in Chicago, IL. I am one of 6 Lead Technicians in the central pharmacy.
Tell us about a time that you made a difference.
When COVID-19 made its way to my hospital, there were a lot of patients that were intubated. With the help of one of our ICU pharmacists, I came up with a plan to make ketamine and midazolam drips for patients in our COVID ICU. We calculated the patient specific drips so that we made enough to last for a 24-hour period and then we delivered them to the units. This helped our nurses to be able to focus on their patients and not have to run over to the pharmacy every few hours.
What pharmacy related issues keep you up at night?
I’m not sure that this issue keeps me from sleeping, but there is definitely a concern with technician pay scale. There are so many excellent technicians in the world of pharmacy; however, we are not always able to show our talents because the compensation is lower than what one can live on. We have done little over the course of my 17 years as a technician to break down these barriers. I believe that with advanced roles there should be advanced compensation.
What makes ICHP great?
ICHP is great because you can meet so many people. It really is an organization that allows people to make great connections and friendships. I have met so many people just working in pharmacy that I normally would not have met.
What initially motivated you to get involved in ICHP?
I was a P1 at Midwestern University when I joined ICHP. I really wanted to go to Legislative Day to get out of class. I ended up joining and that was my first Legislative Day that I attended. Since then, I decided pharmacy school really wasn’t for me, but I became involved in ICHP as a technician member. I was really hooked after I volunteered for my first planning meeting committee.
What is your favorite food?
My favorite food is Mexican. Tacos to be more specific, but I love almost all Mexican food.
Where is your favorite place to vacation?
I don’t have one favorite spot to vacation, but my favorite in the last few years has been Niagara Falls. Not the touristy part of it, but the beauty that is the vineyards and wineries of the area. I never knew that there were vineyards in the region, but when I was researching what to do when I went to Toronto last year, I found out much more about the area. I will definitely return in the future to explore more. ■
ICHP Champions Update
The End of an Era
by Julie Downen, PharmD, BCPS, BCIDP; Antimicrobial Stewardship Coordiator; Memorial Medical Center; ICHP Champions Chair; ICHP Central Region Director
Technology is constantly changing and as it does, ICHP is determined to adapt to meet our members’ needs. In May of this year, the ICHP Board of Directors approved the elimination of the Champions subcommittee. When the Champions program was rolled out in 2009, our Champions served as a liaison between ICHP and pharmacists and technicians throughout Illinois. Over the years, our Champions posted news briefs on pharmacy billboards and set up webinars at institutions. As technology has evolved, ICHP is now able to offer these services directly to each member.
Here is what you can expect:
- Lunch-time webinars will still be held at least every other month. Instead of contacting your worksite champion to participate, you will be able to dial-in to the webinar from anywhere which will provide greater convenience and access for participation. You can still organize a departmental webinar for both live or home study webinars.
- The Champions News Brief will now be called the ICHP News Brief and will continue to be published and distributed to all members.
- As always, all of this content will continue to be available on the ICHP website.
Finally, we want to take a moment to thank all of our Champions (past and present) for the time and energy that they have dedicated to being a Champion. It’s because of you that we were able to sustain and advance such a successful program for more than 10 years. Thank you for all that you do and continue to do for ICHP.
Now, we are all champions! ■
There's Still Time to Step Up!
The Nominations Committee is Nearly Done Calling!
by Scott A. Meyers, Executive Vice President
As I posted in May - every year, ICHP elects new members to its Board of Directors. As existing officers complete their terms, they often move up to higher offices or move on for a variety of reasons not the least being that they’ve completed the highest offices of President-elect, President, and Immediate Past President. So, every year the ICHP Committee on Nominations searches for new leaders to step up to carry on the business of the Council and lead the organization in “Advancing Excellence in Pharmacy!” (That’s ICHP’s mission by the way).
This year is no exception. With Noelle Chapman completing her term as Immediate Past President and several other offices up for election, there are nine offices that will need two candidates to run for each. Below is a list of the offices open for election in the Fall of 2020. All the elected candidates will take office at the 2021 Annual Meeting with the exception of the President-elect, who assumes office immediately. So each new leader will have almost a year to train for their new job and be coached by our current Board Members. You don’t have to run that race unprepared! In addition, job descriptions for each office may be found on the ICHP website at:
www.ichpnet.org/about_us/board_of_directors/job_descriptions/.
We do have at least one candidate for each office but here is a complete list of the offices to be elected this fall:
- President-elect
- Treasurer-elect
- Treasurer (one-year to complete the remainder of Chris Crank’s term)
- Director-elect of the Division of Government Affairs
- Director-elect of the Division of Organizational Affairs
- Director-elect of the Division of Professional Affairs
- Central Region Director-elect
- Northern Region Director-elect
- Southern Region Director-elect
- NPN Chair-elect
If you are interested in running for an office or you would like to know more about an office before committing to run, you may contact the Committee on Nominations Chair, Noelle Chapman at
noelle.chapman@aah.org or Scott Meyers at
scottm@ichpnet.org by the end of August. We hope you are ready to help lead the way for ICHP and Pharmacy! ■
We Need Reasons to Celebrate This Year!
Pharmacy Month, Week and Day are Approaching, so Why Not?
by Scott A. Meyers, Executive Vice President
2020 has left a lot to be desired, am I right?! October is National Pharmacy Month. The third full week in October, the 18th through the 24th, is National Hospital and Health-System Pharmacy Week. And Tuesday, October 20th, is National Pharmacy Technician Day. So even though it is August, get on the ball and start making plans to commemorate all three of these important celebrations! We all know your staff needs a party!
And what’s a pharmacy department to do? A cake is always a good start, but probably not enough for something this auspicious and badly needed. A pizza party for all shifts on any given day during that third week? We’re getting closer. This might not be a good year for an open house in the Department, not to mention IDFPR might frown on that! How about table tents with pharmacy trivia in the cafeteria during the entire month? Simple facts about numbers of prescriptions filled in the U.S. annually, the number of new drugs that were approved by the FDA in 2019, and this year you might even want to talk about treatments for COVID-19! But then again, you might not, but at least you’re cooking if any of these ideas are on your list!
What about tent cards on patients’ meal trays during National Hospital and Health-System Pharmacy Week letting them know that they have access to a pharmacist right there at their bedside if they have questions or concerns about their meds? You might need to invite the dietary supervisors to your pizza party to pull this one off, but it is a great idea!
Whatever you do, don’t miss the opportunity to celebrate! If you’re not the director in your department, ask if you can help or, better yet, coordinate the celebration. Bosses buy ideas if you offer to make them happen. And, if you don’t light the fire, no one will be able to cook! Let’s all make October a month to remember in a year we’d like to forget! ■
Educational Affairs
Post-Pandemic Prevention: The Development of a COVID-19 Vaccine
by Jason Val G. Alegro, PharmD, BCPS, BCIDP; Clinical Pharmacy Specialist, Infectious Diseases, Mount Sinai Hospital & Assistant Professor of Clinical Sciences, Roosevelt University College of Pharmacy; Chelsea Manaligod, PharmD Candidate Roosevelt University College of Pharmacy; Kristen Martinez, PharmD Candidate Roosevelt University College of Pharmacy
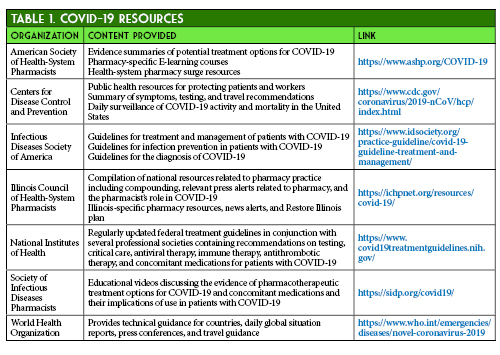
During the first few months of the Coronavirus Disease 2019 (COVID-19) pandemic, the repurposing and development of therapeutic agents has been a major focus of pharmaceutical and clinical research. As the COVID-19 data are continuously evolving and published daily, a list of dynamic resources regarding therapeutic options and public health considerations are provided in Table 1.1-7 Beyond the need for a treatment for this disease, there is also a pressing need for a Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) vaccine.8 With the availability of a SARS-CoV-2 vaccine, there is a greater likelihood of large gatherings and all sectors of the economy re-opening in Illinois.9
In addition to COVID-19 treatments, data are also continuously published surrounding SARS-CoV-2 vaccine candidates. Many vaccine developers are taking the “science by press release” tactic of presenting preliminary, unpublished findings to generate public and economic optimism. This may make it difficult for clinicians to parse through substantive clinical evidence.10 This is compounded with the surge of pre-print publications that obscure the understanding of the current vaccine landscape. This article will review the foundational steps and innovations in SARS-CoV-2 vaccine development, as well as review available data from two vaccine candidates currently in clinical trials.
The Molecular and Immunologic Basis for a SARS-CoV-2 Vaccine
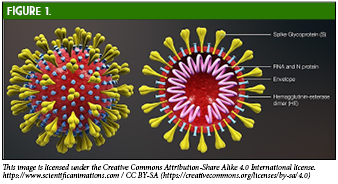
To understand the processes of creating a SARS-CoV-2 vaccine, the virology, immunopathology, and methods for enhancing the immunologic response to this virus must be considered. SARS-CoV-2 enters human cells using angiotensin-converting enzyme (ACE-2) receptors expressed in nasal mucosa, bronchi, and alveolar epithelium, as well as in extrapulmonary tissues such as the kidney, stomach, bladder, and ileum.11 Specifically, the spike (S) glycoprotein found on the outer surface of the virus (Figure 1) exhibits high affinity for human ACE-2 receptors and is responsible for binding, thus allowing virus entry and replication.12,13 The S glycoprotein provides novel major antigenic determinants specific to SARS-CoV-2 and is immunodominant, leading to the production of primarily neutralizing antibodies.14,15,16 These neutralizing antibodies against the receptor binding domain (RBD) of the S protein have been found to be virus-specific inhibitors and are of major interest in vaccine development.17
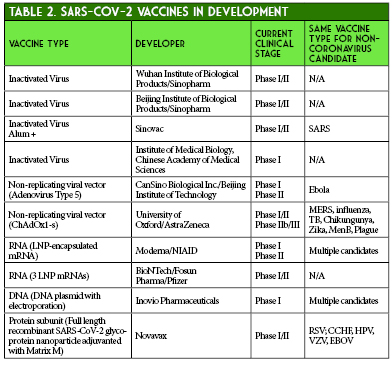
Enhancing immunologic response to SARS-CoV-2 can be achieved through various design pathways, including virus-based vaccines, viral vector vaccines, nucleic-acid vaccines, and protein-based vaccines.18 As of June 9, 2020, there are a total of 10 vaccine candidates undergoing Phase I or Phase II clinical trials. Four of these are inactivated virus vaccines, one is a recombinant protein subunit vaccine, three are nucleic acid vaccines (two RNA and one DNA), and two are non-replicating viral vector vaccines, both using adenovirus as a vector (Table 2).19 The two that will be discussed in detail will be CanSino’s non-replicating adenovirus type 5 (Ad5) vector vaccine and Moderna’s lipid nanoparticle (LNP) encapsulated mRNA vaccine.
CanSino and Moderna’s COVID-19 Vaccine Candidates
CanSino Biologics, based in China, is currently in Phase II trials with their non-replicating Ad5 vectored SARS-CoV-2 vaccine.20 Adenovirus serves as an attractive vaccine vector for several reasons, including its ability to infect both dividing and non-dividing cells, physical and genetic stability, and potential for eliciting strong immune responses. It also has a preference for targeting epithelial cells, which can lead to stimulation of both mucosal and systemic immunity. Some disadvantages include poor immunogenicity in those with prior immunity to adenovirus, and the need for high vaccine doses to elicit an immune response.18,21-23
CanSino’s Phase I trial, recently published in Lancet, explored the safety, tolerability, and immunogenicity of the Ad5 vaccine in subjects in Wuhan, China.24 A total of 108 subjects who had no evidence of COVID-19 infection received one intramuscular injection of low, medium, or high-dose Ad5 vaccine. Adverse events were reported in 30/36 subjects (83%) in the low and medium dose groups and 27/36 subjects (75%) in the high-dose group. Injection site pain occurred in 58 (54%), fever in 50 (46%), fatigue in 47 (44%), headache in 42 (39%), and muscle pain in 18 (17%) recipients. In the high-dose group, 6 subjects (17%) experienced grade 3 adverse events that limited activity in the first 7 days after injection, compared with 2 subjects (6%) in each the low and medium dose groups. T-cell responses peaked at 14 days post-vaccination and antibody responses peaked at day 28, although follow-up ended at day 28. A four-fold increase in antibodies to the receptor-binding domain was demonstrated in 94-100% of patients, and a four-fold increase of antibodies to live-virus in 50-75% of patients. Notably, adults > 60 years old were excluded and only 16% of participants were > 50 years old. The authors concluded that this Ad5 vectored vaccine was tolerable and immunogenic in healthy adults.26 As the company proceeds with their Phase II trial, areas of interest will be evaluating the immunogenicity in older patients more susceptible to severe COVID-19 disease, vaccine durability past 28 days, and further assessing adverse events in a larger, older study population.
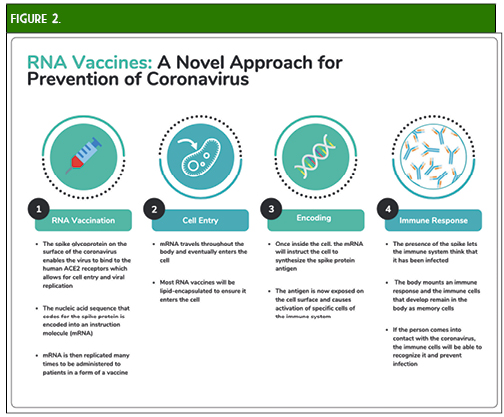
Perhaps the vaccine that is garnering the most interest is the mRNA-1273 vaccine currently being developed by Moderna and the National Institute for Allergy and Infectious Diseases (NIAID).25 This vaccine is an LNP-encapsulated mRNA vaccine expressing a prefusion-stabilized S protein. Advantages of this novel mRNA design include flexible antigen manipulation, high potency (which may confer strong CD4+ and CD8+ responses with one or two doses), and the potential for rapid, cost-effective, large-scale production (Figure 2). Additionally, the lack of viral components will allow production of this vaccine without growing live, highly pathogenic organisms which decreases the risk of contamination or infectious release of SARS-CoV-2 and potential use in bioterrorism. Possible concerns include severe local and systemic inflammatory responses, as well as issues related to stability and RNA degradation which will need to be addressed (e.g., utilizing lipid nanoparticles) in order to take advantage of the quick and efficient in vitro mRNA replication process.25-28
Moderna provided preliminary data on May 18, 2020. The press release provided immunogenicity data for participants receiving two doses of 25 mcg or 100 mcg and only after the first dose of 250 mcg (n = 15 in each cohort). All subjects achieved seroconversion after one dose, and for the 25 mcg and 100 mcg doses, binding antibody levels were similar to those seen in convalescent sera. Eight participants in the 25 mcg and 100 mcg cohorts achieved serum neutralizing antibody titers at or above levels seen in convalescent sera. Three patients in the 250 mcg cohort experienced grade 3 adverse events after the second dose, which were transient and self-resolving. One grade 3 adverse event of erythema around the injection site occurred in one participant in the 100 mcg group. Zero adverse events occurred in participants in the 25 mcg cohort.29 The mRNA-1273 vaccine is currently undergoing Phase II trials comparing 50 mcg and 100 mcg doses with an estimated enrollment of 600 participants.30 A recent press release from Moderna stated that Phase III trials are expected to begin in July and that the study protocol has been finalized. The trial will be a randomized 1:1 (100 mcg dose vs. placebo) trial with an expected enrollment of 30,000 United States participants and a primary endpoint of the prevention of symptomatic COVID-19 infection.31
There are many questions regarding these vaccines that will need to be addressed through clinical trials and long-term follow-up. What is the vaccine effectiveness and durability? Are there any concerns for short and long-term safety particularly with newer vaccine technologies? With the variability seen in patient responses to COVID-19, how will this translate to prevention variability among different demographics, especially in the elderly? How will the vaccine overcome antigenic drift and shift of SARS-CoV-2? What will the role of herd immunity and vaccination rates play in preventing COVID-19 in the future? The successful availability, stability, and distribution of these vaccines will preclude many of these questions. It will only be once a successful vaccine is approved and marketed that we will be able to see its true effect on the population, hopefully leading to an effective post-pandemic reality. ■
_____________________________________________________________________________________________________________________________________________________________________
References:
- American Society of Health System Pharmacists. ASHP COVID-19 Resource Center. https://www.ashp.org/COVID-19 (accessed 2020 Jun 12)
- Centers for Disease Control and Prevention. Information for Healthcare Professionals about Coronavirus (COVID-19). https://www.cdc.gov/coronavirus/2019-nCoV/hcp/index.html (accessed 2020 Jun 12)
- Infectious Diseases Society of America. Infectious Diseases Society of America Guidelines on the Treatment and Management of Patients with COVID-19. https://www.idsociety.org/practice-guideline/covid-19-guideline-treatment-and-management/ (accessed 2020 Jun 12)
- Illinois Council of Health-System Pharmacists. ICHP COVID-19 Resources. https://ichpnet.org/resources/covid-19/ (accessed 2020 Jun 12)
- National Institutes of Health. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. https://www.covid19treatmentguidelines.nih.gov/ (accessed 2020 Jun 12)
- Society of Infectious Diseases Pharmacists. COVID-19 Resources. http://sidp.org/covid19/ (accessed 2020 Jun 12)
- World Health Organization. Coronavirus disease (COVID-19) pandemic. https://www.who.int/emergencies/diseases/novel-coronavirus-2019 (accessed 2020 Jun 12)
- Thometz K. Pritzker Says Illinois on Track to Move to Phase 4, Won’t Lift Restrictions Early. Wttw. 6/10/20.
- Restore Illinois: A Public Health Approach to Safely Reopen Our State. Office of JB Pritzker. https://coronavirus.illinois.gov/sfc/servlet.shepherd/document/download/069t000000BadS0AAJ?operationContext=S1 (accessed 2020 Jun 12).
- Brennan Z, Goldberg D. Coronavirus drugmakers' latest tactics: Science by press release. Politico. 6/8/20.
- Jin Y, Yang H, Ji W, et al. Virology, Epidemiology, Pathogenesis, and Control of COVID-19. Viruses. 2020;12(4):372. Published 2020 Mar 27. doi:10.3390/v12040372,
- https://it.wikipedia.org/wiki/File:3D_medical_animation_corona_virus.jpg
- Zhang H, Penninger, JM, Li Y, et al. Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: molecular mechanisms and potential therapeutic target. Intensive Care Med. 46: 586–590. doi: 10.1007/s00134-020-05985-9
- Zheng M, Song L. Novel antibody epitopes dominate the antigenicity of spike glycoprotein in SARS-CoV-2 compared to SARS-CoV. Cell Mol Immunol. 2020. 17; 536-538. https://doi.org/10.1038/s41423-020-0385-z
- Prekumar L, Segovia-Chumbez B, Jadi R, Martinez D, Raut R. The receptor binding domain of the viral spike protein is an immunodominant and highly specific target of antibodies in SARS-CoV-2 patients. Science Immunology. 2020; 5: 1-14. DOI: 10.1126/sciimmunol.abc8413
- Mukherjee S, Tworowski D, Detroja R, Mukherjee SB, Frenkel-Morgenstern M. Immunoinformatics and Structural Analysis for Identification of Immunodominant Epitopes in SARS-CoV-2 as Potential Vaccine Targets.Vaccines (Basel). 2020. E290; 1-17. doi: 10.3390/vaccines8020290.
- Ju B, Zhang Q, Ge J, et al. Human neutralizing antibodies elicited by SARS-CoV-2 infection [published online ahead of print, 2020 May 26]. Nature. 2020;10.1038/s41586-020-2380-z. doi:10.1038/s41586-020-2380-z
- Callaway E. The race for coronavirus vaccines: a graphical guide. Nature. 2020;580(7805):576‐577. doi:10.1038/d41586-020-01221-y
- World Health Organization. DRAFT landscape of COVID-19 candidate vaccines–30 April 2020. https://www.who.int/who-documents-detail/draft-landscape-of-covid-19-candidate-vaccines. (accessed 2020 June 13)
- A Phase II Clinical Trial to Evaluate the Recombinant Vaccine for COVID-19 (Adenovirus Vector) (CTII-nCoV). ClinicalTrials.gov Identifier: NCT04341389. https://clinicaltrials.gov/ct2/show/NCT04341389
- Amanat F, Krammer F. SARS-CoV-2 Vaccines: Status Report. Immunity. 2020;52(4):583‐589. doi:10.1016/j.immuni.2020.03.007
- Robert-Guroff M. Replicating and non-replicating viral vectors for vaccine development. Curr Opin Biotechnol. 2007;18(6):546‐556. doi:10.1016/j.copbio.2007.10.010
- Ahi YS, Bangari DS, Mittal SK. Adenoviral vector immunity: its implications and circumvention strategies. Curr Gene Ther. 2011;11(4):307‐320. doi:10.2174/156652311796150372
- Zhu FC, Li YH, Guan XH, et al. Safety, tolerability, and immunogenicity of a recombinant adenovirus type-5 vectored COVID-19 vaccine: a dose-escalation, open-label, non-randomised, first-in-human trial. Lancet. 2020; 395: 1845-1854.
- Safety and immunogenicity study of 2019-nCoV Vaccine (mRNA-1273) to prevent SARS-CoV-2 Infection. ClinicalTrials.gov Identifier: NCT04283461. https://clinicaltrials.gov/ct2/show/NCT04283461
- Zhang C, Maruggi G, Shan H, Li J. Advances in mRNA Vaccines for Infectious Diseases. Front Immunol. 2019; 10: 1-13.
- Wang F, Kream RM, Stefano GB. An Evidence Based Perspective on mRNA-SARS-CoV-2 Vaccine Development. Med Sci Monit. 2020;26:e924700. Published 2020 May 5. doi:10.12659/MSM.924700
- Diamond MS, Pierson TC. The Challenges of Vaccine Development against a New Virus during a Pandemic. Cell Host Microbe. 2020;27(5):699‐703. doi:10.1016/j.chom.2020.04.021
- Moderna Announces Positive Interim Phase 1 Data For Its Mrna Vaccine (Mrna-1273) Against Novel Coronavirus. May 18, 2020. Press release.
- Dose-Confirmation Study to Evaluate the Safety, Reactogenicity, and Immunogenicity of mRNA-1273 COVID-19 Vaccine in Adults Aged 18 Years and Older. Clinicaltrials.gov Identifier: NCT04405076.
- Moderna Advances Late-Stage Development of its Vaccine (mRNA-1273) Against COVID-19. June 11, 2020. Press release.
Educational Affairs
Blast from the Past: Convalescent Plasma for COVID-19
by Katie Miles, PharmD Clinical Assistant Professor/Academic Detailing Pharmacist; University of Chicago College of Pharmacy; Heather Ipema, PharmD, BCPS Clinical Assistant Professor; Drug Information Group; University of Chicago College of Pharmacy
Introduction
Coronavirus disease 2019, better known as COVID-19, is a respiratory infectious disease that has rapidly spread throughout the world.1,2 The first few cases were recognized and reported in Wuhan, China in December 2019. On March 11, 2020, the World Health Organization (WHO) declared COVID-19 a pandemic.1-3 COVID-19 is caused by a novel coronavirus called severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).1,2 Most cases are considered mild (≥80%) with the remaining 14% of cases reported as serious and 5% as critical.2 Some cases (10%) require hospitalization for COVID-19 pneumonia with 10% of those cases necessitating intensive care unit (ICU) admission due to acute respiratory distress syndrome (ARDS).
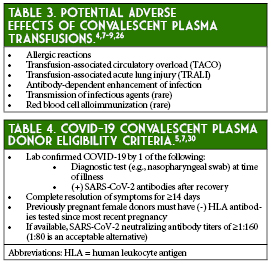
At this time, there are no Food and Drug Administration (FDA)-approved treatment options for COVID-19 and no medication has been shown to be safe and effective for this infection.4,5 One potential treatment that has been used and is regulated by the FDA as an investigational product is COVID-19 convalescent plasma (herein after referred to as “convalescent plasma”).5,6 The Infectious Diseases Society of America (IDSA) recommends convalescent plasma in hospitalized patients in the setting of a clinical trial; though, the authors note a knowledge gap for this recommendation.2 The National Institutes of Health (NIH) also address convalescent plasma in their COVID-19 treatment guideline, but are unable to recommend for or against its use (graded as a strong recommendation based on expert opinion [AIII]) due to a lack of data and theoretical risks (e.g., antibody-dependent enhancement of infection and transfusion-associated lung injury [TRALI]).4
COVID-19 Convalescent Plasma Product Information
Convalescent plasma is serum collected from patients who have recovered from COVID-19 and that have produced antibodies to SARS-CoV-2.4,5,7-9 These antibodies exert their therapeutic effect by neutralizing the virus in the recipient.8,9 Additionally, the antibodies can activate complement and initiate antibody-dependent cellular toxicity and phagocytosis. Convalescent plasma provides short-term passive immunity as either prophylaxis or for treatment to reduce viral infectivity. Due to the large population of recovered COVID-19 patients, convalescent plasma is a readily available resource for prophylaxis or treatment.
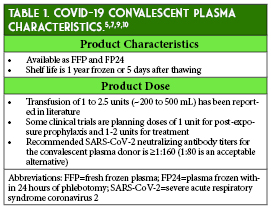
The dose and preferred timing for administration of convalescent plasma during the course of a COVID-19 infection has not been established.7 Dosing of convalescent plasma in general has been widely variable throughout the history of its use as post-exposure prophylaxis and/or treatment in several viral infections (e.g., polio and Ebola), including respiratory infection outbreaks, including the 2009-2010 H1N1 influenza virus pandemic, 2003 SARS-CoV-1 epidemic, and 2012 Middle East Respiratory Syndrome coronavirus (MERS-CoV) epidemic.5,9 Once convalescent plasma is administered, the duration of the circulating antibodies’ therapeutic effect is unclear and dependent on the antibody amount given, but may last weeks to months.8,9 Table 1 provides additional characteristics of COVID-19 convalescent plasma.
Review of COVID-19 Convalescent Plasma Studies for the Treatment of COVID-19
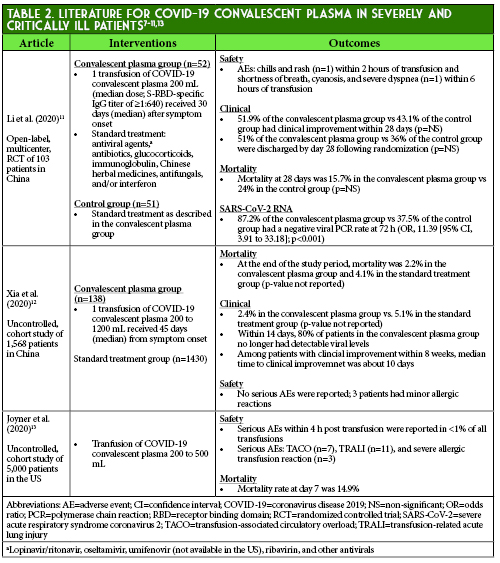
At this time, the efficacy and safety of COVID-19 convalescent plasma are still under investigation.4,5,7 Most of the available data consists of small observational studies from China and the US.4,7,11-22 A systematic review of the lower quality evidence concluded that effectiveness of convalescent plasma in hospitalized COVID-19 patients was uncertain based on inconsistentencies in the reported results.23 More recently, mortality data from 1 small randomized trial and 2 larger cohort studies have been published (Table 2).11-13 Much of the literature is limited by lack of control groups and administration of several other concurrent therapies, which complicates the determination of convalescent plasma’s true effect in COVID-19.12-22, 24
The two completed randomized controlled trials were conducted in China and the Netherlands and several controlled clinical trials are underway in the US and internationally.7,11,20 The primary analysis of the randomized controlled trial by Li et al. did not find statistically significant differences in clinical outcomes between patients that received convalescent plasma in addition to standard of care and patients that received standard of care alone; however, the trial was terminated early due to decreasing new COVID-19 cases in Wuhan, China resulting in an underpowered study.11,25 A subgroup analysis found an association between convalescent plasma and clinical improvement in severely ill patients, but not those with life-threatening COVID-19. Additionally, convalescent plasma was associated with a significantly higher rate of SARS-CoV-2 RNA clearance as compared to standard of care patients.
Safety Concerns of COVID-19 Convalescent Plasma
Convalescent plasma transfusion has several theoretical risks associated with its use based on known risks associated with standard plasma transfusions that are therefore not unique to this specific product (Table 3).4,7-9, 26 These potential adverse effects have not been fully established for COVID-19 convalescent plasma.7 The historical data from SARS and MERS suggest that convalescent plasma use in coronavirus infections is potentially safe, although two case reports of possible convalescent plasma-induced TRALI events have been described in a patient with Ebola and a patient with MERS.8,9, 26-28 Possible transfusion-related adverse events that resolved with corticosteroid treatment were reported in two patients in the randomized controlled trial by Li et al.11 Additionally, Joyner et al. reported limited cases of transfusion-associated circulatory overload (TACO), TRALI, and severe allergic reactions, but no significant adverse events were reported in most of the COVID-19 studies or in several previous SARS-associated coronavirus and severe influenza studies.13, 14, 16-22, 29
Access to COVID-19 Convalescent Plasma in the United States
The FDA has published recommendations for the use of convalescent plasma.5,7,30 The guidance covers COVID-19 convalescent plasma access, collection, labeling, record keeping, and patient eligibility criteria. In the United States, COVID-19 convalescent plasma is restricted to three pathways: clinical trials, an expanded access investigational new drug (IND) application, and a single patient emergency investigational new drug (eIND) application. The FDA recommendations for investigational convalescent plasma contain more detail on recipient eligibility.5 Mayo Clinic is the leading institution collecting and providing COVID-19 convalescent plasma for the National Expanded Access Program.31 A dedicated website provides a map of the registered sites for COVID-19 convalescent plasma administration (https://www.uscovidplasma.org/family).
COVID-19 Convalescent Plasma Donation
The FDA has set forth certain eligibility criteria for COVID-19 convalescent plasma donation (Table 4).5,7,30 Convalescent plasma can be donated at blood donation centers or through the American Red
Cross.31,32 Blood donation centers can be located through the American Association of Blood Banks (AABB) website.32 One donor of COVID-19 convalescent plasma is estimated to provide enough plasma to treat two to three patients.33
Conclusions
COVID-19 convalescent plasma is an investigational treatment for COVID-19 that has shown potential benefit. Randomized controlled trials are needed to further establish efficacy and safety. As therapy and
evidence continue to evolve, the use of this investigational product is likely to increase. ■
References
- Fauci AS, Lane HC, Redfield RR. Covid-19 - navigating the uncharted. N Engl J Med. 2020;382:1268-1269.
- Bhimraj A, Morgan RL, Shumaker AH, et al. Infectious Diseases Society of America guidelines on the treatment and management of patients with COVID-19. Clin Infect Dis. 2020.
- World Health Organization. WHO director-general's opening remarks at the media briefing on COVID-19 - 11 March 2020. https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020 (accessed 2020 July 10).
- National Institutes of Health. COVID-19 treatment guidelines (updated May 12, 2020). https://covid19treatmentguidelines.nih.gov/therapeutic-options-under-investigation/host-modifiers-immunotherapy/ (accessed 2020 July 10).
- Food and Drug Administration. Recommendations for investigational COVID-19 convalescent plasma (updated May 1, 2020). https://www.fda.gov/vaccines-blood-biologics/investigational-new-drug-ind-or-device-exemption-ide-process-cber/recommendations-investigational-covid-19-convalescent-plasma (accessed 2020 July 10).
- Food and Drug Administration. Coronavirus disease 2019 (COVID-19) resources for health professionals (updated July 6, 2020). https://www.fda.gov/health-professionals/coronavirus-disease-2019-covid-19-resources-health-professionals (accessed 2020 July 10).
- American Society of Health-System Pharmacists. Assessment of evidence for COVID-19-related treatments (updated July 2, 2020). https://www.ashp.org/-/media/assets/pharmacy-practice/resource-centers/Coronavirus/docs/ASHP-COVID-19-Evidence-Table.ashx (accessed 2020 July 10).
- Casadevall A, Pirofski LA. The convalescent sera option for containing COVID-19. J Clin Invest. 2020;130:1545-1548.
- Bloch EM, Shoham S, Casadevall A, et al. Deployment of convalescent plasma for the prevention and treatment of COVID-19. J Clin Invest. 2020.
- American Red Cross. Coronavirus (COVID-19) convalescent plasma clinician information. https://www.redcrossblood.org/donate-blood/dlp/plasma-donations-from-recovered-covid-19-patients/clinician-registration.html (accessed 2020 July 10).
- Li L, Zhang W, Hu Y, et al. Effect of convalescent plasma therapy on time to clinical improvement in patients with severe and life-threatening COVID-19: a randomized clinical trial. JAMA. 2020.
- Xia X, Kening L, Lingxiang W, et al. Improved clinical symptoms and mortality on severe/critical COVID-19 patients utilizing convalescent plasma transfusion. Blood. 2020.
- Joyner M, Wright RS, Fairweather D, et al. Early safety indicators of COVID-19 convalescent plasma in 5,000 patients. medRxiv. 2020.
- Mehmet AE, Sarici A, Berber I, et al. Life-saving effect of convalescent plasma treatment in COVID-19 disease: clinical trial from eastern Anatolia. Transfus Apher Sci. 2020.
- Hartman W, Hess AS, Connor JP. Hospitalized COVID-19 patients treated with convalescent plasma in a mid-size city in the midwest. medRxiv. 2020.
- Hegerova L, Gooley T, Sweerus KA, et al. Use of convalescent plasma in hospitalized patients with COVID-19 – case series. Blood. 2020.
- Salazar E, Perez KK, Ashraf M, et al. Treatment of COVID-19 patients with convalescent plasma. Am J Pathol. 2020.
- Olivares-Gazca JC, Priesca-Marin JM, Ojeda-Laguna M, et al. Infusion of convalescent plasma is associated with clincial improvement in critically ill patients with COVID-19: a pilot study. Rev Invest Clin. 2020;72:159-164.
- Liu STH, Lin H, Baine I, et al. Convalescent plasma treatment of severe COVID-19: a matched control study. medRxiv. 2020.
- Gharbharan A, Jordans CCE, GeurtsvanKessel C, et al. Convalescent plasma for COVID-19. A randomized clinical trial. medRxiv. 2020.
- Duan K, Liu B, Li C, et al. Effectiveness of convalescent plasma therapy in severe COVID-19 patients. Proc Natl Acad Sci U S A. 2020;117:9490-9496.
- Zeng QL, Yu ZJ, Gou JJ, et al. Effect of convalescent plasma therapy on viral shedding and survival in COVID-19 patients. J Infect Dis. 2020.
- Valk SJ, Piechotta V, Chai KL, et al. Convalescent plasma or hyperimmune immunoglobulin for people with COVID-19: a rapid review. Cochrane Database Syst Rev. 2020;5:Cd013600.
- Roback JD, Guarner J. Convalescent plasma to treat COVID-19: possibilities and challenges. JAMA. 2020.
- Casadevall A, Joyner MJ, Pirofski LA. A randomized trial of convalescent plasma for COVID-19-potentially hopeful signals. JAMA. 2020.
- Tiberghien P, de Lamballerie X, Morel P, et al. Collecting and evaluating convalescent plasma for COVID-19 treatment: why and how? Vox Sang. 2020.
- Chun S, Chung CR, Ha YE, et al. Possible transfusion-related acute lung injury following convalescent plasma transfusion in a patient with Middle East Respiratory Syndrome. Ann Lab Med. 2016;36:393-395.
- Mora-Rillo M, Arsuaga M, Ramírez-Olivencia G, et al. Acute respiratory distress syndrome after convalescent plasma use: treatment of a patient with Ebola virus disease contracted in Madrid, Spain. Lancet Respir Med. 2015;3:554-562.
- Mair-Jenkins J, Saavedra-Campos M, Baillie JK, et al. The effectiveness of convalescent plasma and hyperimmune immunoglobulin for the treatment of severe acute respiratory infections of viral etiology: a systematic review and exploratory meta-analysis. J Infect Dis. 2015;211(1):80-90.
- Food and Drug Administration. Guidance for industry: investigational COVID-19 convalescent plasma (May 26, 2020). https://www.fda.gov/regulatory-information/search-fda-guidance-documents/investigational-covid-19-convalescent-plasma (accessed 2020 July 10).
- Mayo Clinic. COVID-19 expanded access program. https://www.uscovidplasma.org/ (accessed 2020 July 10).
- Mayo Clinic. How do individuals donate plasma for this protocol? https://www.uscovidplasma.org/donate (accessed 2020 July 10).
- American Society of Hematology. COVID-19 and convalescent plasma: frequently asked questions (updated June 8, 2020). https://www.hematology.org/covid-19/covid-19-and-convalescent-plasma (accessed 2020 July 10).
Educational Affairs
Review of Capsaicin's Use for the Management of Cannabinoid Hypermesis Syndrome
by Carol DesLauriers, PharmD, DABAT Assistant Vice President, Illinois Health and Hospital Association; Anthony Renzoni, PharmD; PGY2 Emergency Medicine Pharmacy Resident; Rush University Medical Center; Anthony Burda, RPh, DABAT Clinical Toxicologist, Illinois Poison Center
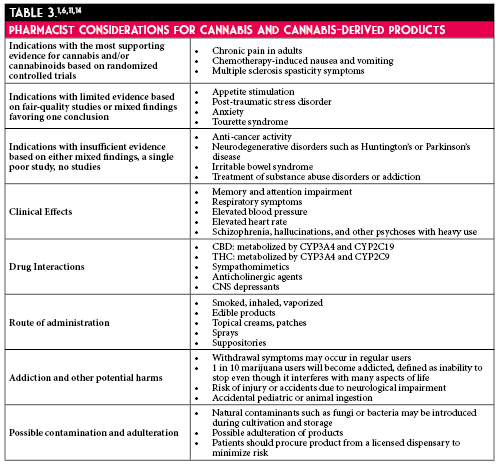
According to the United Nations World Drug Report, cannabis was the most used drug in the world in 2017, accounting for 183 million users.1 Legalization of cannabis has been increasing since 2012. In May 2019, the Illinois General Assembly passed HB-1438, Cannabis Regulation & Tax Act, which legalized recreational cannabis for use and sale. As of January 1, 2020, Illinois residents aged 21 years and older can legally purchase and possess up to 30 grams of plant material, edibles totaling no more than 500 mg of tetrahydrocannabinol, and 5 grams of cannabis concentrate products.2 With legalization, pharmacists should be aware of the signs, symptoms, and management of Cannabinoid Hyperemesis Syndrome (CHS).
CHS is characterized by recurrent, paroxysmal episodes of nausea, vomiting, and abdominal discomfort in chronic cannabis users.3 Patients present to Emergency Departments seeking treatment for intractable vomiting. There are three phases of CHS which include prodrome, hyperemesis, and recovery. During the prodrome phase, patients may experience early morning nausea, fear of vomiting, and abdominal discomfort. The hyperemesis phase can last up to 24 hours where patients have nausea, vomiting, and abdominal pain. Diagnostic criteria for CHS includes weekly use of cannabis, long-term cannabis use (>1 year), severe cyclic nausea and vomiting (SCNV), relief of symptoms with hot showers, abdominal pain, age <50 years, weight loss of >5 kg, and morning predominance of symptoms.5,6 CHS should be included in the differential diagnosis for patients presenting with gastrointestinal symptoms of unknown origin and in those with social history notable for chronic cannabis use who report resolution of symptoms with hot showers. Obtaining an in-depth social history for patients with CHS can potentially avoid additional imaging and diagnostic testing from being performed while in the ED.
The complex pathophysiology of SCNV involves the chemoreceptor trigger zone of the medulla oblongata, also known as the area postrema.6 This structure is found outside of the blood-brain barrier and is sensitive to blood and cerebrospinal fluid-born chemicals.6 Emesis begins with increased salivation, deep respirations, closure of the glottis, and relaxation of the pyloric sphincter. From there, retroperistalsis begins from the small intestine to the stomach and contraction of the abdominal muscles, resulting in emesis.7 The pathophysiology of CHS involves the endocannabinoid system. Endogenous cannabinoids bind to G protein-coupled cannabinoid receptors CB1. These receptors are found in the central nervous system and gastrointestinal (GI) tract and contribute to emesis. They modulate gastric secretion, motility, inflammation, and sensation.6 Activation of these receptors by endogenous cannabinoids inhibit the hypothalamic-pituitary-adrenal axis and central nervous system response to stressful stimuli.8 Early use of cannabis may lead to anti-nausea effects, but repeated use of cannabis can lead to desensitization of these receptors resulting in vomiting.
Several medications are often trialed in the Emergency Department for the management of CHS. Dopamine antagonists (metoclopramide, promethazine, haloperidol, droperidol, prochlorperazine) inhibit dopamine in the chemoreceptor trigger zone and have prokinetic effects on motor function of the GI tract. Serotonin antagonists (ondansetron) work on enterochromaffin cells in the GI tract, which release serotonin in response to noxious substances, to inhibit afferent sensory neurons. Benzodiazepines may also have a role in CHS due to their inhibition of medullary and vestibular nuclei associated with SCNV. These are considered standard therapies trialed for CHS but are often observed to be ineffective in clinical practice.
Other therapies have been reported for CHS and are largely driven by case reports and small case series. Capsaicin, a chemical found in chili peppers, binds to transient receptor potential vanilloid-1 (TRPV1) receptors which are found near CB1 receptors in the GI tract and medullary vomiting center. These receptors are activated by low pH and high temperature and regulate the release of substance P. Hot showers are theorized to work due to dose-dependent hypothermic effect of cannabinoids binding to CB1 receptors of the hypothalamus. Another theory is that hot showers cause cutaneous vasodilation which alters core temperature, splanchnic circulation leading to decreased abdominal discomfort.4
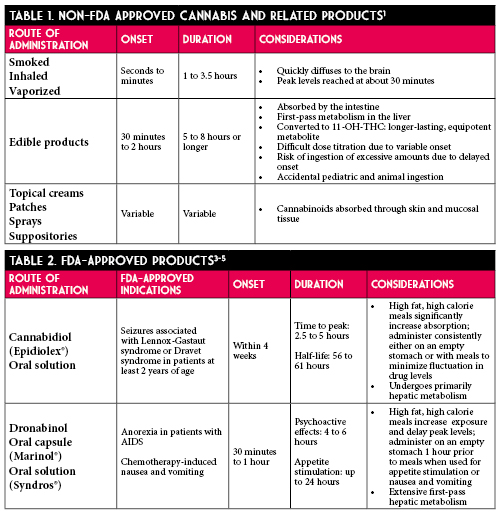
Despite only low methodological quality of evidence, some institutions are trialing capsaicin cream as an anti-emetic.10 Capsaicin cream is marketed for temporary relief of pain due to arthritis and strains/sprains. It is available over-the-counter in concentrations of 0.025% to 0.1%. It is reasonable to recommend capsaicin 0.075% cream to be applied to the abdomen and upper arms 3-4 times a day. Capsaicin does not have comparative efficacy data to support its use as a first-line agent. An advantage to capsaicin is its ease of administration as a cream since other anti-emetics, in the oral dosage forms, are contraindicated in active vomiting. If patients are discharged on the medication, there are several critical counseling points to ensure patient safety. Common side effects include local skin reactions at the application site and sneezing due to inhalation of dried cream. Patients should avoid application to the eyes, genitourinary regions, sensitive skin, and mucous membranes. To avoid exposure to hands and fingers, patients should wash hands after application and consider wearing gloves or using finger cots during application. Patients should also be advised to avoid application of heat or occluding the application site.18 Most importantly, patients should be advised abstinence from cannabis is the most effective treatment for CHS.
A systematic review including three case reports11,12,13 and two case series14,15 found capsaicin to be effective in 18 patients. Two retrospective cohort studies did not find capsaicin to demonstrate a significant benefit on ED length of stay. In one of the retrospective cohort studies, capsaicin administration was associated with a non-significant prolonged time to ED discharge (51.1 minutes longer; 95% CI: -17.6 to 119.9) and non-significantly reduced rate of hospital admission (33.3% vs 61.9%; P=0.055).16 In the other retrospective cohort study, ED length of stay was non-significantly reduced in CHS patient visits during which capsaicin was administered compared with those in which it was not (179 vs 201 minutes, P=0.33).17 Most patients experienced clinical resolution of CHS after 1 dose. Time to resolution of symptoms may have ranged from within 30 minutes up to 11 hours after use.11-15
With states passing laws allowing the sale of cannabis for recreational use, health care providers can expect to see an increase in use and possibly an increase in CHS. When standard anti-emetic agents fail to treat CHS, patients may try topical capsaicin. Although weak literature exists, pharmacists should be cognizant of its place in therapy and should be able to recommend safe dosing and administration. ■
References
- United Nations Office on Drugs and Crime. World Drug Report 2017. https://www.unodc.org/unodc/en/press/releases/2017/June/world-drug-report-2017_-29-5-million-people-globally-suffer-from-drug-use-disorders--opioids-the-most-harmful.html (accessed 2020 Jun 23).
- Illinois General Assembly. Cannabis Regulation & Tax Act Springfield, IL. 2019. http://www.ilga.gov/legislation/ilcs/ilcs5.asp?ActID=3992&ChapterID=35 (accessed 2020 Jun 23).
- Allen JH, de Moore GM, Heddle R, Twartz JC. Cannabinoid hyperemesis: cyclical hyperemesis in association with chronic cannabis use. Gut. 2004; 53: 1566-1570.
- Richards JR. Cannabinoid Hyperemesis Syndrome: Pathophysiology and Treatment in the Emergency Department. J Emerg Med. 2018; 54(3): 354-363.
- Simonetto DA, Oxentenko AS, Herman ML, Szostek JH. Cannabinoid hyperemesis: a case of 98 patients. Mayo Clin Proc. 2012; 87(2): 114-119.
- Sorenson CJ, DeSanto K, Borgelt L, et al. Cannabinoid hyperemesis syndrome: diagnosis, pathophysiology, and treatment – a systematic review. J Med Toxicol. 2017; 13(1): 71-87.
- Hendren G, Aponte-Feliciano A, Kovac A. Safety and efficacy of commonly used antiemetics. Expert Opin Drug Metab Toxicol. 2015; 11: 1753-67.
- Abalo R, Vera G, Lopez-Perez AE, Martinez-Villaluenga M, Martin-Fontelles MMI. The gastrointestinal pharmacology of cannabionoids: focus on motility. Pharmacology. 2012; 90(1-2): 1-10.
- Carvalho AF, Van Bockstaele EJ. Cannabinoid modulation of noradrenergic circuits: implications for psychiatric disorders. Prog Neuropsychopharmacol Biol Psychiatry. 2012; 38: 59-67.
- Lapoint J, Meyer S, Yu CK, Koenig KL, Lev R, Thihalolipavan S, Staats K, Kahn CA. Cannabinoid Hyperemesis Syndrome: Public Health Implications and a Novel Model Treatment Guideline. West J Emerg Med. 2018; 19(2): 380-386.
- Roman F, Llorens P, Burillo-Putze G. Topical capsaicin cream in the treatment for cannabinoid hyperemesis syndrome [in Spanish]. Med Clin (Barc). 2016; 147(11): 517-518.
- Moon AM, Buckley SA, Mark NM. Successful treatment of cannabinoid hyperemesis syndrome with topical capsaicin. ACG Case Rep J. 2018; 5:e3.
- Sharma U. Cannabis hyperemesis syndrome. BMJ Case Rep. 2018. doi:10.1136/bcr-2018-226524.
- Dezieck L, Hafez Z, Conicella A, et al. Resolution of cannabis hyperemesis syndrome with topical capsaicin in the emergency department: a case series. Clin Toxicol (Phila). 2017; 55: 908-913.
- Graham J, Barberio M, Wang GS. Capsaicin cream for treatment of cannabinoid hyperemesis syndrome in adolescents: a case series. Pediatrics. 2017; 140: e20163795.
- McCloskey K, Goldberger D, Rajasimhan S, McKeever R, Vearrier D. Use of topical capsaicin cream for the treatment of cannabinoid hyperemesis syndrome. Clin Toxicol. 2017; 55: 828-829.
- Wagner S, McLaughlin J, Hoppe J, Zuckerman M, Schwarz K. Efficacy and safety of topical capsaicin for cannabinoid hyperemesis syndrome in the emergency department. Clin Toxicol. 2018; 56: 982.
- Zostrix [package insert]. Ann Arbor, MI: Akron Consumer Health; 2020.
Educational Affairs
A review of Medical Marijuana and the Pharmacist's Role
by Chloe Majkowski, PharmD Clinical Pharmacist, Oncology Northwestern Medicine Huntley Hospital; Amanda N. Seddon, PharmD, BCPS, BCOP Assistant Professor of Pharmacy Practice Midwestern University, Chicago College of Pharmacy; Justin D. Fisher, PharmD Clinical Pharmacy Specialist, Hematology/Oncology/Cellular Therapy Rush University Medical Center
With increasing legalization at the state level for both medical and recreational marijuana, pharmacists are in a position to educate patients and other healthcare professionals on the risks and potential benefits of cannabis. While there continues to be much controversy on the clinical use of marijuana, pharmacists are an important resource in understanding disease states in which it may provide potential benefit, as well as risks, including drug-drug interactions and adverse effects.
The earliest written records of human cannabis use date back to the 6th century BC, but our understanding of its physiologic effects has only more recently become apparent.1 More than 104 chemical compounds that act on cannabinoid receptors have been identified in Cannabis. It is important to note the differences between cannabis and associated products. Cannabis is a botanical product, and most commonly utilized in the form of marijuana, or dried leaves of the cannabis plant. Cannabinoids are chemical compounds found in cannabis that act on cannabinoid receptors. Tetrahydrocannabinol (THC) and cannabidiol (CBD) are the most abundant cannabinoids and biologically active compounds found in cannabis. THC is a psychoactive compound associated with the commonly experienced “high,” and CBD is a non-psychoactive compound that tends to produce a relaxing effect. The most well-known species within the Cannabis genus are Cannabis sativa, which generally has higher concentrations of THC, and Cannabis indica, which usually contains more CBD.
Pharmacology and Physiologic Effects of Cannabis
Pharmacology
The human endocannabinoid system consists of two types of receptors, CB1 and CB2.1 CB1 receptors are primarily concentrated in the brain and central nervous system, while CB2 receptors are mostly found in peripheral organs, especially those associated with the immune system. CBD has a low affinity for both CB1 and CB2, but acts as an allosteric modulator at opioid receptors and is a known serotonin receptor agonist.1,2 THC binds with greater affinity to CB1 and CB2, which results in the typical effects associated with marijuana use.1
Administration
Various routes of administration are available for cannabis and associated products.1 Route of administration should be carefully considered due to implications of varying pharmacokinetic factors, such as the risk of overdose with edible products due to their delayed onset of action. Notable differences in onset, duration, and special considerations are summarized in Tables 1 and 2.
Metabolism and Drug Interactions
Both THC and CBD are metabolized by cytochrome P450 (CYP) 3A4 enzymes.6 Additionally, THC is metabolized by CYP2C9, and CBD by CYP2C19. Because of this, patients with altered CYP450 enzymes who are poor metabolizers may experience increased or prolonged effects of these compounds. Drug interactions with concomitant use of CYP450 inhibitors or inducers may also be relevant. Further, the physiologic effects of these compounds may be increased when used in combination with sympathomimetic drugs, CNS depressants, or anticholinergic agents.
Physiologic Effects
Acute effects of cannabis include enhanced sensitivity to stimuli, altered perception of time, increased appetite, decreased short-term memory, dry mouth, and impaired perception and motor skills.1 Very high blood levels of THC may result in panic attacks, paranoid thoughts, or hallucinations. Rarely, users may experience cannabinoid hyperemesis syndrome, a difficult to treat phenomenon characterized by cyclic vomiting associated with chronic cannabis use.7 The dosage of THC as well as individual variability in absorption and metabolism may influence the intensity and duration of intoxication.1 Additionally, desensitization with sustained use of cannabis may occur as chronic cannabis use leads to down-regulation of CB1 receptors, which may be reversed by abstinence.
Laws and Regulations
Marijuana remains a schedule I substance under the Controlled Substances Act due to its high potential for dependence and lack of a widely accepted medical use, making distribution of cannabis a federal offense.8 Because federal law prohibits prescriptions for marijuana, many states allow medical marijuana “recommendations” or “referrals” rather than prescriptions. In Illinois, the medical cannabis pilot operation was initiated in 2014, and over 80,000 people in Illinois participated in the program. The program was later made permanent, taking effect January 1, 2020.
In order to purchase medical marijuana in Illinois, patients must receive a certification from their provider confirming a qualifying debilitating condition.9 There are currently over 50 qualifying conditions in Illinois including cancer, chronic pain, migraines, irritable bowel syndrome, and seizures. Once certification is obtained, the patient may purchase a registry ID card which is valid for 3 years.8,10 Under this registry, patients may purchase up to 2.5 ounces of medical cannabis during a 14-day period, and may grow up to 5 plants at home. The new law also allows students to use medical cannabis under the supervision of a school administrator or nurse on school property; previously, a student could only receive medical marijuana from a parent or guardian on school grounds. Of note, recreational marijuana was also legalized in Illinois under a law that took effect January 1, 2020. Illinois is the 11th state to legalize recreational marijuana for adult use. Lastly, over-the-counter products containing CBD extracted from cannabis are available in all 50 states.11,12 It is only legal to sell or purchase these products if there is less than 0.3% THC in the product.
Medical Literature
High-quality evidence exists for FDA-approved products cannabidiol (Epidiolex®) and dronabinol (Syndros®, Marinol®). Epidiolex is a purified form of CBD used in the setting of childhood epilepsy,
specifically Lennox-Gastaut Syndrome (LGS) and Dravet Syndrome (DS). Studies found a 41-44% reduction in seizures versus placebo in patients with LGS and DS.13 Additionally, synthetic THC derivatives, nabilone and dronabinol, are widely accepted treatments for refractory chemotherapy-induced nausea and vomiting, though nabilone has since been discontinued.14
The FDA has not approved cannabis for treatment of any disease or condition and sites the need for additional clinical trials to assess safety and effectiveness.9 While no randomized controlled trials exist for the use of cannabis in the medical literature, there are smaller studies that may help guide our treatment decisions. A comprehensive review of available literature was conducted by the National Academies of Sciences, Engineering, and Medicine, and the group published a public health report in 2017 on the health effects of cannabis and cannabinoids.1 Several findings were considered conclusive or with substantial evidence for use. First, the Academy found that cannabis or cannabinoids are effective for the treatment of chronic pain in adults. Second, oral cannabinoids are considered effective antiemetics for the treatment of chemotherapy-induced nausea and vomiting. Lastly, oral cannabinoids are effective for improving patient-reported multiple sclerosis spasticity symptoms. However, the effects of cannabis and cannabinoids are modest for all of these conditions, and there is inadequate information to assess effects for all other indications that were evaluated. More recent prospective studies, although small, have demonstrated benefit of sublingual THC oil for neuropathic pain, and inhaled cannabis vapor for chronic fibromyalgia pain.15,16
Pharmacist’s Role in Patient Management and Cannabis Use
Pharmacists play an important role in educating patients that cannabis is not a replacement for evidence-based pharmacotherapy, and more research is needed to confirm the safety and efficacy of cannabis and related products. When considering cannabis products for clinical use, pharmacists should discuss both risks and benefits with the patient and provider. Patients who may benefit most would be those with chronic pain, chemotherapy-induced nausea and vomiting, and multiple sclerosis spasticity. Additionally, pharmacists should review patient medication lists and educate providers on potential drug-drug interactions. For instance, some patients receiving cancer treatment may also be receiving an azole antifungal, which may increase the concentration of both THC and CBD due to CYP3A4 inhibition. On the other hand, certain anticonvulsants (i.e. carbamazepine) may reduce THC and CBD concentrations, resulting in decreased effects of the cannabis product. Additionally, it is important to consider patient-specific factors when determining whether cannabis or cannabinoids would be useful, and what dosage form may be preferable. Patients with uncontrolled blood pressure or those with memory or attention deficits may not be the best candidates for cannabis-derived products. For patients with underlying respiratory issues, edible products may be preferred over inhaled formulations. Pharmacists should also educate patients on the risk of impairment and addiction, as well as obtaining products from a licensed source to minimize the risk of adulteration or contamination. Continued follow-up with the patient and monitoring for efficacy and side effects should be implemented if a cannabis product is initiated.
Considerations for pharmacists managing patients who choose to use cannabis and cannabis-derived products are summarized in Table 3.
As cannabis and related products become more widely used in both the recreational and medical settings, it is meaningful for pharmacists to have an adequate understanding of these substances. Open conversations on the risks and benefits, comprehensive medication and social histories, and routine monitoring are steps pharmacists can take as the use of these products continues to increase.
Additional Resources
Educational Affairs
Call for Posters!
Are you working on a project that others could learn from?
Please consider sharing the outcomes with your colleagues at the poster session during the ICHP/MSHP Spring Meeting March 19-20, 2021 in Collinsville, IL! This is a great opportunity to share innovative ideas with others and learn about trends in Illinois health-system pharmacies.
Categories/Presentations/Eligibility
Submission:
Members wishing to submit a poster should use the online submission form. Be sure to click "Submit" after completing your form. The deadline for submissions is January 10, 2021. Please direct any questions to Trish Wegner at
TrishW@ichpnet.org.
Deadlines:
Submission deadline is January 10, 2021. Authors will be notified of acceptance of their poster via email in February, 2021.
Criteria determined by the Division of Educational Affairs ■
Features
Opioid Task Force - CPE Opportunity!
Emergency Department (ED) Dispensing of Naloxone Take-Home Kits
Feature Article
by Marc McDowell, PharmD, BCPS; Emergency Medicine Clinical Specialist; Emergency Medicine PGY-2 Residency Director; Advocate Christ Medical Center; Oak Lawn, IL
Introduction, Purpose, and Goals of the Program
The opioid crisis is a public health epidemic without boundaries. The devastation brought by the misuse of prescription or illicitly-obtained opioids is unparalleled within this generation. Opioid-related deaths rose to a record 47,600 in 2017 in the United States, constituting a 600% increase in less than two decades.1 In 2018, over 72,000 people in North America died of opioid-related overdoses. Approximately 2,200 of these fatalities occurred in Illinois.2 The deaths attributable to opioid misuse are twice as high as all Chicagoland homicides or motor vehicle fatalities during the same year.3
Unfortunately, the case fatality rate is expected to climb exponentially over the coming decade. It is currently estimated that for every fatal overdose, 30 non-fatal overdoses will occur.4 By this approximation, Illinois’ citizens suffered approximately 66,000 opioid overdoses in the past calendar year. Although the disease state of opioid use disorder (OUD) is multifactorial, initial opioid exposure is one of the major contributors to this epidemic.5 Approximately 16% of patients receiving more than a one-week supply of opioids and 6% receiving a one-day supply reported continued use after one year. For this reason, a focus on safe prescribing practices is necessary.
In addition to the tragic loss of life and the burden to the healthcare system, medical care costs are another victim of this crisis. Emergency Department (ED) visits related to opioid overdose continues to rise with a 65.5% increase seen from 2016 to 2017.The mean hospital cost for an opioid overdose treated solely in the ED is $504, but this increases drastically to $11,731 for an inpatient admission. This cost climbs to over $20,000 if a stay in intensive care is required.6 This introduces a large financial stressor on healthcare systems and may limit their ability to provide other necessary services to the community.
Opioid Use Disorder (OUD) is often linked with the socioeconomically and functionally marginalized populations. These patients often have poorer access to primary medical care and may rely on the ED as their main point of contact for health care. This creates a distinct opportunity for healthcare professionals within the ED to intervene and address the current opioid epidemic. Staggeringly, after being discharged from an ED for an opioid overdose 1% of patients die within a month, and over five percent die within one year.7 ED prescribers and pharmacists are afforded the unique ability to identify those suffering and those at risk of suffering from this disease. From this vantage point, a number of harm reduction strategies, initiatives, and programs can be implemented to effectively assist those suffering with this disease.
Recently, dispensing naloxone to the general population from harm reduction agencies has begun to take root in at-risk communities. These programs are endorsed by several professional medical organizations for high-risk patients.8 Specifically, the Centers for Disease Control and Prevention endorses the creation, utilization, and expansion of naloxone distribution programs. Naloxone, a potent mu-opioid receptor reversal agent, is frequently administered by pre-hospital emergency medical services (EMS), hospital staff, and more recently the general public. A growing body of evidence has demonstrated the feasibility and success of naloxone distribution programs.9 Research by Abouk et al demonstrated that mortality can be directly decreased by dispensing naloxone to at-risk patients being discharged from a health system or ED.10,11
Appropriate selection of at-risk patients remains vital in combating this epidemic. These patients present along a continuous and challenging spectrum. Utilizing an appropriate screening tool is paramount to capturing and assisting all instances of potential OUD. This ranges from the candidate who presents to the ED via emergency medicine services for a repeated life-threatening opioid overdose to more subtle yet just as dangerous scenarios. For example, a patient with underlying mental illness presenting with a moderate to major traumatic injury may be in a precarious position if they require concomitant atypical antipsychotics and opioid therapy for analgesia.12 The adoption of a tool to screen patients for opioid dependence may be a logical approach to ensure safe prescribing practices. The Rapid Opioid Dependence Screen (RODS) is an 8-item validated tool used to assist and measure opioid dependence (Table 1).13 The ease of use of RODS makes it an ideal instrument for assessing current dependence in the ED setting. However, determining active dependence and potential for opioid abuse are similar but distinct. The Opioid Risk Tool (ORT) is a brief, self-reportable screening tool designed for use with adult patients in primary care settings (Table 2).14 Depending on the practice setting and patient population, identifying and using the appropriate questionnaire or combination thereof can broaden a program’s impact on potential OUD patients.
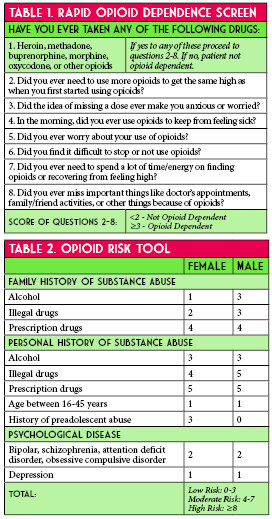
Unfortunately, many patients with opioid use disorder do not seek appropriate resources prior to their first overdose. Recent literature suggests that only 18% of naloxone prescriptions are filled and picked up after discharge leaving many patients untreated.15 As previously mentioned, the ED is a major touch point in the opioid battle. Given the prevalence of unmet substance abuse needs among patients in the ED and the increasing frequency of drug-related visits the ED acts as a vital link to appropriate medical care for at-risk patients. In September of 2017, the Illinois Department of Public Health (IDPH) issued a standing order authorizing pharmacists to dispense naloxone for reversing potential opioid overdoses without a direct prescription.
Our health-system recognized that pharmacists and pharmacy technicians were poised to translate these data into a tangible and successful program. Our goal was the implementation of a systematic and comprehensive process involving free distribution of naloxone from the ED. We aimed to accomplish this in a simple and cost-efficient manner to provide a potentially lifesaving intervention to an at-risk demographic.
Design and Implementation
In May 2018 an interdisciplinary task force of pharmacists and physicians met to address the issue of local OUD in the community (Figure 1). The discharge naloxone program was conceptualized in late May. One of the initial steps of the task force was to identify key stakeholders needed to implement a successful ED naloxone discharge kit process. Providers from emergency medicine, pain management, pediatrics, information technology pharmacy, behavioral health, inpatient substance withdrawal unit, and ED nursing were jointly collaborated to leverage their expertise and clinical experience. Goals for this team were to provide take-home naloxone, at no cost, to patients at risk of OUD or their family members.
Subsequently, supply of medications and necessary supplies were addressed. In partnership with a local community harm reduction agency, the pharmacy department designed and obtained naloxone kits via combination of donation and grant contributions. Each kit contained 0.4mg/1ml naloxone vials, small three mL syringes, and intramuscular needles in triplicate, as well as community substance abuse outreach information. Kits were contained in a small protect from light bag large enough to affix a prescription label. Kits were stored in central pharmacy.
The team developed several documents, training pathways, and poster materials to aid in the education of all parties involved with this project. Primarily the documents focused on the availability of the take-home kit, patient eligibility for a kit, and work-flow process to obtain a kit. These were posted throughout the ED in patient rooms, physician charting areas, nurse workspaces, and the ED pharmacy work station. Additionally, a nine minute training video was shared among all staff detailing the importance of OUD and the effectiveness of outpatient naloxone. Development, training, and procurement of medication occurred over the next four months.
Optimal workflow was operationalized with an ED pharmacist identifying a potential patient at risk of OUD and outpatient opioid overdose (Figure 2). This was done by assessing a RODS and ODT score, evaluating patients with home regimens on abnormally large doses or long durations of opioids, those with history a of OUD, those presenting to the ED for an acute opioid overdose, as well as family or friends of those presenting with any of the above patients. The ED pharmacist clinical acumen was used to determine abnormal doses and duration of opioid outpatient prescriptions. The pharmacist would then engage in open dialogue with the at-risk patient and, if appropriate, their caregiver, family, or friend regarding OUD and the danger of an opioid overdose. This conversation would offer the take-home naloxone kit at no charge. Additionally the ED pharmacist would provide counseling regarding naloxone or any other pertinent patient medications. If the patient was interested in receiving a kit, the pharmacist would then share the same nine minute training video with the patient. This video detailed the ideal process for utilization of the naloxone kit. In a stepwise fashion the video reviewed when to administer naloxone, how to draw up the medication from the vial into a syringe and administer as an intramuscular injection, how often to re-administer, and when to call 911. The ED pharmacist would then enter the order to dispense a kit in the electronic medical record and ensure the kit delivery to the patient. The pharmacist would then document in the medical record that the patient received the kit. All dispensing is reported to the Illinois Prescription Monitoring Program in compliance with Illinois state law.
Preliminary Results
Project go-live was approximately six months after conceptualization on September 6th, 2018. A total of 212 kits, which included 636 doses of naloxone were distributed in the 20 months since initiation (Figure 3). Some trends are observed from this data. The initial months of the program utilization was relatively low, averaging only five kits dispensed per month. Over the course of the subsequent 15 months a drastic increase in distribution occurred. This is evidenced by over 60% of all kits being dispensed in the final six months of data collection. This demonstrates an exponential growth of the program. Expanded utilization is attributable to multiple factors.
Initial hesitation by physicians to recommend naloxone at discharge was anecdotally reported. However, as the program progressed, familiarity, awareness, and comfort seemed to increase for all members within the department. Additionally, ED pharmacy coverage expanded during the final months of data collection. This allowed our team to capture more of these at-risk patients and increase overall distribution. On average, our ED treats approximately 15 opioid overdose patients monthly. By this estimation we were able to capture and intervene in over 70% of this population. Based on the upward trend of kit distribution and the spread of the epidemic, we anticipate that this program will continue to grow in the coming months.
Unfortunately, due to the nature of the disease state and pharmacologic intervention, obtaining objective patient-centered outcomes is difficult. Measuring kit utilization, lives saved, reduced utilization of EMS, admissions, and intensive care needs is cumbersome, if not impossible to quantify. However, the costs associated with each of those components of the healthcare system are substantially higher compared to three doses of naloxone.
Hurdles and Lessons Learned
Despite the eventual success of this program, several barriers challenged the key stakeholders during development. Provider and administrator concern regarding the legal liability surrounding the take-home
kit was an initial challenge. Educating stakeholders and discussing the full extent of the IDPH standing order alleviated this hesitation. An additional layer of complexity was realized regarding state pharmacy law concerning outpatient medication dispensing from an inpatient hospital setting. Specifically, if this kit were dispensed from the ED, what labeling would be appropriate or necessary? A concise review of Illinois State Law and Acts by a recent peer-reviewed publication by Eswaran and colleagues is an excellent tool for navigating this issue.16 This was overcome by ensuring NDC, LOT, and expiration dates were all tracked by the inpatient pharmacy department. Similar labels utilized for sexual assault post-exposure prophylaxis were affixed to the physical kit.
Physician and nursing resistance was also a preliminary challenge. This was in part due to lack of familiarity with a harms reduction strategy and a fear of encouraging unhealthy behaviors. With further education and time, providers witnessed not only the benefit of such a program by their peers who were utilizing the kits, but also the gratitude from at-risk patients. The increase in dispensing rates is evidence that adoption of the program became more widespread over time.
Funding and securing supplies were initial concerns for the program as well. As mentioned, providing the kits at no charge was one of our primary goals. It was not feasible to bill the insurance for the dispensed product considering the setting of the ED. Thus our options were donating inpatient supply as charity care, obtaining outside funding via grants or donations, or utilizing our 340B program. Ultimately, we were able to obtain assistance from a local harm-reduction organization. However close consideration was given to charity care as the cost-benefit of saving just one ED visit would have financed the program for well over a year.
While reliance on the ED pharmacist to assess, consent, counsel, and obtain the kit for the patient reinforced our professions aptitude and necessity, it also proved a barrier. Due to limited and variable ED pharmacist coverage undoubtedly some candidates for the kit fell through the cracks. Although our services were available the majority of hours in the day, seven days a week, access to naloxone kits for an at-risk patients should not be impacted by a lack of resources. Opportunities for improvement include providing education for nursing and physician staff to duplicate these responsibilities. Pharmacy technicians served a vital role in this program as well. They not only delivered kits to the ED but also manually batched the kits.
summary and future goals
Our novel program has impacted the lives of the patients as well as their families. By dispensing 212 kits in a relatively brief amount of time, our team of pharmacists have elevated the quality of life in the at-risk community in our area at no additional cost to the institution. This clearly demonstrates that clinical pharmacists are effective leaders and stewards in the fight against the opioid epidemic. Pharmacy technicians are important to the success of such programs; assisting with kit creation and timely distribution being two essential tasks they are able to facilitate.
Currently, the naloxone discharge kit program is being expanded to the inpatient areas of our institution. This will help capture at-risk patients who are directly admitted to the hospital who may have bypassed the ED or those who are diagnosed with OUD later in their hospitalization. There is a strong need for appropriate treatment of opioid use disorder among our patients admitted to the hospital for other medical reasons. By having this kit and education available to inpatient participants, we can address this disease state in a cost-effective and proactive manner. In the future we hope to expand this program across several other institutions in our healthcare system to offer a new standard of care within our shared communities.
Sustainability is key for our service to continue to be offered to our at-risk community. Continuing to secure grant funding is paramount to our success. We are currently aggressively tracking metrics on patients with OUD who present to the ED and how frequently they participate in the program and obtain appropriate follow-up. ■
_____________________________________________________________________________________________________________________________________________________________________
References
- United Nations Office on Drugs and Crime. World Drug Report 2017. https://www.unodc.org/unodc/en/press/releases/2017/June/world-drug-report-2017_-29-5-million-people-globally-suffer-from-drug-use-disorders--opioids-the-most-harmful.html (accessed 2020 Jun 23).
- Illinois General Assembly. Cannabis Regulation & Tax Act Springfield, IL. 2019. http://www.ilga.gov/legislation/ilcs/ilcs5.asp?ActID=3992&ChapterID=35 (accessed 2020 Jun 23).
- Allen JH, de Moore GM, Heddle R, Twartz JC. Cannabinoid hyperemesis: cyclical hyperemesis in association with chronic cannabis use. Gut. 2004; 53: 1566-1570.
- Richards JR. Cannabinoid Hyperemesis Syndrome: Pathophysiology and Treatment in the Emergency Department. J Emerg Med. 2018; 54(3): 354-363.
- Simonetto DA, Oxentenko AS, Herman ML, Szostek JH. Cannabinoid hyperemesis: a case of 98 patients. Mayo Clin Proc. 2012; 87(2): 114-119.
- Sorenson CJ, DeSanto K, Borgelt L, et al. Cannabinoid hyperemesis syndrome: diagnosis, pathophysiology, and treatment – a systematic review. J Med Toxicol. 2017; 13(1): 71-87.
- Hendren G, Aponte-Feliciano A, Kovac A. Safety and efficacy of commonly used antiemetics. Expert Opin Drug Metab Toxicol. 2015; 11: 1753-67.
- Abalo R, Vera G, Lopez-Perez AE, Martinez-Villaluenga M, Martin-Fontelles MMI. The gastrointestinal pharmacology of cannabionoids: focus on motility. Pharmacology. 2012; 90(1-2): 1-10.
- Carvalho AF, Van Bockstaele EJ. Cannabinoid modulation of noradrenergic circuits: implications for psychiatric disorders. Prog Neuropsychopharmacol Biol Psychiatry. 2012; 38: 59-67.
- Lapoint J, Meyer S, Yu CK, Koenig KL, Lev R, Thihalolipavan S, Staats K, Kahn CA. Cannabinoid Hyperemesis Syndrome: Public Health Implications and a Novel Model Treatment Guideline. West J Emerg Med. 2018; 19(2): 380-386.
- Roman F, Llorens P, Burillo-Putze G. Topical capsaicin cream in the treatment for cannabinoid hyperemesis syndrome [in Spanish]. Med Clin (Barc). 2016; 147(11): 517-518.
- Moon AM, Buckley SA, Mark NM. Successful treatment of cannabinoid hyperemesis syndrome with topical capsaicin. ACG Case Rep J. 2018; 5:e3.
- Sharma U. Cannabis hyperemesis syndrome. BMJ Case Rep. 2018. doi:10.1136/bcr-2018-226524.
- Dezieck L, Hafez Z, Conicella A, et al. Resolution of cannabis hyperemesis syndrome with topical capsaicin in the emergency department: a case series. Clin Toxicol (Phila). 2017; 55: 908-913.
- Graham J, Barberio M, Wang GS. Capsaicin cream for treatment of cannabinoid hyperemesis syndrome in adolescents: a case series. Pediatrics. 2017; 140: e20163795.
- McCloskey K, Goldberger D, Rajasimhan S, McKeever R, Vearrier D. Use of topical capsaicin cream for the treatment of cannabinoid hyperemesis syndrome. Clin Toxicol. 2017; 55: 828-829.
- Wagner S, McLaughlin J, Hoppe J, Zuckerman M, Schwarz K. Efficacy and safety of topical capsaicin for cannabinoid hyperemesis syndrome in the emergency department. Clin Toxicol. 2018; 56: 982.
- Zostrix [package insert]. Ann Arbor, MI: Akron Consumer Health; 2020.
____________________________________________________________________________________________________________________________________________________________
Another Perspective
Publons: A Tool to Help with Predatory Journals and How Pharmacists Can Help Stem the Tide
by Milena Murray, PharmD, MSc, BCIDP, AAHIVP; Associate Professor of Pharmacy Practice, Midwestern University Chicago College of Pharmacy; HIV/ID Clinical Pharmacist, Northwestern Memorial Hospitall; Alfredo Traversa, PharmD PGY-2 Infectious Diseases Pharmacy Resident, Edward Hines, Jr. VA Hospital
Disclaimer: “Opinions expressed by authors of this article are their own and are not necessarily shared by ICHP or its members. ICHP will publish these articles from time to time to direct you to topics that may be of interest to you and to stimulate discussion on potentially controversial issues of the day.” The authors of this article are not affiliated with Publons and have not received any type of compensation for this work.
Predatory publishing is a threat to evidence-based medicine. Journals accept manuscripts along with oftentimes exorbitant authors’ fees with the promise of rapid publication.1 In addition, editorial board membership may be misrepresented. After acceptance, there is often no further system of checks and balances for plagiarism or ethical approvals.2 Some predatory journals even send manuscripts for “peer review”; however, the comments are not provided to the authors and edits are never made.3 Some are easy to spot, sending emails with calls for research articles that include sentences such as, “Your eminent research would greatly enhance our respected issue.” and there is even a Twitter™ parody account to follow the most humorous calls for publication (@GreetingsForDay). However, many researchers are shocked to learn that they have submitted to predatory journals.3 There are extensive lists available to “weed” out predatory journals, however, these journals adapt quickly to “warning signs” on these lists.4
Pharmacists who perform research and who peer review have a duty to the profession and the greater research community to avoid submitting to and reviewing for these predatory journals. But as noted, it is possible to inadvertently interact with these journals. This is where the use of Publons can help both individuals and the research community.
What is Publons?
Publons (
www.publons.com) is a dynamic peer review platform that provides recognition for completed peer reviews. Publons verifies, tracks, and promotes member contributions to academic journals. Publons' mission is to "speed up science by harnessing the power of peer review". Launched in 2012, it already has more than 175,000 active members. Once a Publons profile is complete you can upload reviews or simply just have the name of the journal included on your profile. When a finished review has been accepted by a journal, a copy of the confirmation email may be forwarded to
reviews@publons.com. Alternatively, there are journals that will now proactively ask if you would like to add the review to your Publons profile. The review is then verified and added to your profile within a few days.
Enhancing Quality, Credible Reviewers
In addition to the issues of predatory journals, there is a growing epidemic of peer review fraud, in which some individuals will give glowing reviews on an article to boost its chances of being published. Publons wishes to overcome this concerning trend by verifying and authenticating a member’s profile and only allowing verified profiles to upload reviews. Editors may mark reviews as “excellent”, rewarding reviewers who have helped deliver quality reviews that advance the profession. Editors may also find qualified peer reviewers based upon credentials through the Publons dashboard.
Enhancing Quality, Credible References
Because Publons tracks peer reviews, analyses can be performed to see if any reviews have been submitted for predatory journals. Reports and feedback can then be provided to help stem the tide of reviews for these types of journals. Education and intervention have been identified as important techniques to drive down the number of publications in predatory journals.2
Individualized Reports, An Added Bonus for Pharmacy Faculty
For faculty, tracking peer reviews is often not in the forefront of our minds throughout the year. Publons provides a method to easily track service done through peer reviews. Using a curriculum vitae (CV) to display peer review contributions for countless journals can be uninspiring. With Publons, members can easily export relative content and statistics to enhance their professional portfolio and demonstrate service. Reports can be filtered by several different criteria and a link to your overall peer review service can be generated to add to your CV. For those thinking ahead to promotion, you can filter by a specific date range to add a very professional looking report to your dossier, complete with your profile picture. As I filled out my evaluation this year and eventually reached the question that included peer reviews, I was immediately able to know exactly how many I had completed within the past 12 months.
Awareness and avoidance of predatory journals should be important education topics for all pharmacists, especially those considering research and publication. Authors and reviewers are encouraged to develop a process to make an informed decision regarding the validity of a potential journal for article submission or article peer review. This process should be based upon ethical and validated publishing processes and the journal communications with both authors and reviewers. ■
2020 ICHP Annual Meeting
Going digital!
Join us!
College Connection
Midwestern University Chicago College of Pharmacy
Mental Health and Wellness: Building a Better Tomorrow
College Connection
by Irum Khan, PS-4; ICHP President; Midwestern Universty Chicago College of Pharmacy

According to the Active Minds Foundation, 39% of students in college experience a significant mental health issue, and two thirds of students with anxiety or depression do not seek treatment.1 Since mental health is an essential component of a strong emotional, psychological, and social wellbeing, our chapter here at Midwestern University strives to create more events geared towards advocating for mental health. It is important to break the stigma of mental health issues and provide students with the resources to help others and provide tips to stay positive and prevent these issues from occurring. To help with this cause, our chapter partnered with the National Alliance of Mental Illness (NAMI) to provide a “Mental Health First Aid” session that introduces participants to vital risk factors and warning signs of mental health struggles. This session prepares students to interact with a patient in crisis and connect them with the most appropriate help.
During the 8-hour Mental Health First Aid session, students were trained to identify, understand, and respond to signs of mental illness. This course spells out a clear, five-step action plan to help individuals in crisis connect with the right professionals and peers. This type of support network provides the best chance to avert mental health crises and connect those in need to key resources, opening the door to a positive change. In addition, this training session builds an understanding of the impact we can make and dives into appropriate support. Participants are taught to recognize if a close friend, family member, or colleague is struggling and aid in communication to make positive changes and respond with appropriate empathy. In addition to recognizing mental illness, the session also helps participants recognize substance use disorder.
Upon completing the training session, students were awarded a Mental Health First Aid Training Certification from NAMI. The training certification is a nationally recognized certification, and students will be able to implement what they gained from the session in their communities. The 35 students that participated in the NAMI Mental Health First Aid session stated that they appreciated ICHP for shining a light on mental health issues. This session made students feel empowered and trained to turn a dark situation into a positive one. By adopting these types of programs, we can change procedures, break stigmas, and impact the community in an effective way.
Our chapter plans to continue adding more events focusing on health and wellness. Currently, we are planning to conduct a “Burnout Panel”. Burnout is a state of mental, emotional, and physical exhaustion caused by excessive and prolonged stress. Feeling burnt out can cause harmful mental effects and hinder academic ability. This panel will consist of current pharmacy residents, and the event will start by each panelist talking about their own experiences with burnout in their respective programs. Then, panelists will answer questions we have prepared pertaining to dealing with burnout, and tips to avoid burnout. After, we plan to open it up to the student audience for a Q&A session. Our chapter aims to teach students tips on how to prevent feeling overwhelmed and to show students how to handle situations when they feel their mental health is compromised.
It is important for organizations to not only help students grow professionally but also to help them grow mentally. Chapters can contact programs like NAMI and brainstorm other events to advocate for mental health in their own communities. The more wellness is talked about the more our peers will feel comfortable seeking help. As Michelle Obama once said, “At the root of this dilemma is the way we view mental health in this country. Whether an illness affects your heart, your leg or your brain, it’s still an illness, and there should be no distinction.”2 ■
References
- Active Minds. Statistics. https://www.activeminds.org/about-mental-health/statistics/ (accessed 2020 Mar 25).
- Obama White House. Speeches and Remarks. https://obamawhitehouse.archives.gov/the-press-office/2015/03/04/remarks-first-lady-change-direction-mental-health-event (accessed 2020 Mar 25).
Roosevelt University College of Pharmacy
New E-Board, New Goals
College Connection
by Ali Naserallah, PS-2, President Roosevelt University College of Pharmacy
As we transition into our new roles as the Roosevelt University College of Pharmacy SSHP E-board Class of 2022, one of our major goals this year is to promote residency-focused events for the school. From past SSHP members attending Midyear, it became apparent that many of our students were unaware of possible post-graduate opportunities, specifically in residency. The SSHP chapter ended the school year by hosting an online event on Zoom led by upperclassmen to discuss considerations when applying to a pharmacy residency. This discussion, as well as a Q & A session afterwards, enabled students to interact and ask future pharmacy residents questions about what they did to set themselves up for success during the application process. The future residents gave thorough responses and advice to the attendees on what to expect in the upcoming years of their pharmacy school career. Many students were interested in the event itself as well as securing a residency after pharmacy school for additional training. We would like our fellow students to recognize the different types of pharmacy residencies and, of course, help guide them with future career decisions.
For this upcoming school year, our first plan is to host the annual lab coat fundraiser to assist first year pharmacy students in obtaining lab coats. Additionally, we plan to collaborate with other university organizations to host volunteer events for our communities. Furthermore, we hope to organize events that focus on mentoring students to help sharpen their clinical skills and better prepare them for experiential rotations. We would also like to invite guest speakers with clinical pharmacy experience to discuss their roles in the healthcare world and to help students better understand the opportunities within clinical pharmacy. Past events hosted by SSHP such as journal clubs, residency week, and curriculum vitae workshops will also be considered for this school year. We are all excited to be part of something special and are eager to start the next school year as part of the new SSHP E-board! ■
Rosalind Franklin University College of Pharmacy
Thriving in the New Normal
College Connection
by Monica Salabun, P4 Rosalind Franklin University College of Pharmacy
After a school year full of unexpected adjustments, the students of Rosalind Franklin University (RFU) College of Pharmacy have made great strides to adapt to the new normal in which we are all now living. Though our time on campus did not come to a close as we would normally expect for an academic year, we were all very grateful for the hard work from our dedicated faculty to ensure our learning experiences remained the same. While time slowed down for a little bit, it quickly picked back up for the students completing their P3 year, who have now transitioned into P4 Advanced Practice Pharmacy Experiences (APPEs).
Clinical health system APPEs are a core component for students; one that most look forward to experiencing. Thankfully, with the help of temperature checks and symptom screenings every morning, select remote capabilities, as well as incredibly accommodating preceptors, our P4 students were able to continue with their inpatient rotations in Chicago area hospitals. Although accessibility to patients is variable depending on the given rotation, students are now taking the initiative to make the necessary changes and offering ideas to improve workflow to ensure the best care for patients during the pandemic. Today’s learning environment is one for the books, and students are excited to be back in full swing to continue on our path of becoming the best future clinical pharmacists we can be!
Students who are continuing on in their didactic studies have also had to make many adjustments in order to move forward and stay on track. Most students had to finish the academic year juggling assignments, exams, and a demanding work schedule amid the global pandemic. A P3 student and the incoming RFU ICHP Chapter President, Nadine Alwawi, found herself working harder than ever to successfully complete her final exams while taking on additional shifts as a Pharmacy Technician at Advocate Christ to aid the increased dispensing demand. She came across many changes in treatment regimens, one of which included the initiation of the medication remdesivir for the treatment of patients with COVID-19. Nadine’s experience and passion for health-systems pharmacy practice inspires current members and will definitely attract new members in the upcoming academic year. She is eager to get ICHP members at RFU involved in the organization.
None of us can know what to fully expect for the Fall Quarter and the upcoming academic year at this point in time. Regardless of the delivery platform, our entire chapter looks forward to hosting many university-wide activities to inform students about the developments and breakthroughs that have been made for the future of pharmacy during these uncertain times. We are also looking forward to having fun and innovative fundraisers to help support our initiatives. We can’t wait to see what is in store for the organization! ■
Southern Illinois University Edwardsville School of Pharmacy
Meet the Executive Board
College Connection
by Sara Gardner, P2, President-Elect; Southern Illinois University at Edwardsville School of Pharmacy
With the start of a new school year, let’s give a warm welcome to our 2020-2021 Executive Board! We can’t wait to get involved with the exciting events that SSHP has to offer.
President: Justin Shiau, P3, served as our President-Elect last year. He has been an invaluable member of our team, and we’re proud to have him as our President! Justin also serves as a Senator for our Student Government, and he is the Director of Communications for IPhO. Justin is excited about our chapter’s Residency “Happy Hour” events!
Fun Facts: Justin loves to be creative and makes lots of arts and crafts. He is also fluent in English and Chinese, and can even speak some French!
President-Elect: Sara Gardner, P2, is excited for another year with SSHP! She served as our P1 Liaison last year, and she loved helping P1 students get involved with this great organization. Sara has a strong interest in clinical pharmacy, and she can’t wait to begin pursuing a residency. She is looking forward to starting integrated therapeutics courses this semester!
Fun Facts: Sara is a huge animal lover; she has two cats and a dog. She also plays the drums!
Vice President: Renae Oelrich, P3, currently works at a hospital, and she aspires to continue working in the hospital setting as a pharmacist. Renae became involved with SSHP to learn about ways to reach her goals in health systems pharmacy. Renae also serves as the Secretary for Rho Chi.
Fun Facts: Renae has a two-year-old beagle named Chester, and she makes the best chocolate cupcakes ever!
Secretary: Lesley Swick, P3, aspires to pursue a residency after graduation. She appreciates that SSHP makes it a mission to prepare its members for post-graduation opportunities. Lesley also serves as the Vice President for Rho Chi, the Membership Vice President for APhA-ASP, and a Student Ambassador!
Fun Facts: Lesley has never been on a plane, but she loves to travel. She would love to visit Australia!
Treasurer: Hadizat Olagunju, P2, has always wanted to pursue a career as a pharmacist in a clinical practice setting, and she aspires to work in a hospital as a pharmacist. She knows that being a member of SSHP will help build a bridge toward achieving this goal. Hadizat is looking forward to all of the fun activities and events that SSHP has planned this year!
Fun Facts: Hadizat has three siblings, and she is the oldest. She loves to dance and travel!
Fundraising Chair: Brittany Holshouser, P2, is excited to be a member of SSHP! She would love to pursue a career as a pharmacist in a hospital setting, and she hopes to match with a residency program during her 4th year. Brittany also serves as the President-Elect for APhA-ASP. Brittany is looking forward to the day that we can safely return to campus and be in a classroom setting again!
Fun Facts: Brittany had braces for seven years straight, and she was accidentally named after Britney Spears!
Membership Chair: Janki Vyas, P3, served as our Community Chair last year, and she did a great job! She is interested in pursuing a career in hospital pharmacy after graduation, and she knows that the networking opportunities made available by SSHP will have a positive impact on her career. Janki also serves as the Director of National Engagement for IPhO. She is excited to meet the new members of our E-Board, and to help think of new project ideas!
Fun Facts: Janki is a vegetarian, and she has never tasted meat in her entire life!
Professional Practice Chair: William Stephens, P3, has developed an interest for hospital pharmacy both from his experience as a student technician at a hospital, and from taking therapeutics courses. SSHP has been another way for him to connect to the world of clinical pharmacy. Will is excited to finish up his therapeutics courses and take his first steps toward pursuing a residency!
Fun Facts: Will loves to play disc golf, fish, and play video games!
Community Chair: Miguel Alvarez, P3, plans to pursue a residency program after graduating from SIUE. Miguel also serves as the President of PPAG. He is looking forward to taking a pediatrics course this year, as well as attending the ASHP Midyear Clinical Meeting and the PPAG Annual Meeting!
Fun Facts: Miguel is half Filipino, and he likes to play bass guitar!
ICHP Liaison: Korinne Frankford, P3, knew before she began pharmacy school that she wanted to be involved with hospital pharmacy in some aspect of her career. That led her to join SSHP! Kori has served as the Secretary of the Class of 2022. She is looking forward to getting to know more about SSHP outside of the student level.
Fun Facts: Kori has an undergraduate degree in Spanish, and although she has not been outside of the continental United States, she hopes to travel more and perhaps live in another country!
ASHP Liaison: Bobby Dedo, P3, is interested in pursuing a residency and ultimately a clinical pharmacy position, and he knows that SSHP provides opportunities to better prepare for that endeavor. Bobby serves as the Historian for Rho Chi, and is also the Operation Immunization Chair for SNPhA. He is looking forward to playing an active role in patient care and other volunteer events set to be offered throughout the upcoming year!
Fun Fact: Bobby can play the saxophone! ■
More
Upcoming Events
ICHP Live Webinar
August 26, 2020
Topic: How to Structure a Pharmacy Technician Advancement Program
Speaker: Kelsey Bridgeman, PharmD, MSGH
Accredited for pharmacy technicians
ICHP Live Webinar
September 9, 2020
Topic: IV Antibiotics in IV Drug Users
Speaker: Radhika Polisetty, PharmD, BCIDP, BCPS, AQ-ID, AAHIVP
Accredited for health-system pharmacists
2020 ICHP Annual Meeting
October 2-3, 2020
We're going digital!
More details to follow via e-mail.
ICHP Live Webinar
November 24, 2020
Topic: Interoperability for Combating the Opioid Crisis: ILPMP
Speaker: Adam Bursua, PharmD, BCPS
More details to follow at a later date.
Please keep an eye out for affiliate emails!
We've got some great home study webinars planned for the coming weeks!
Welcome New Members!
ICHP is happy to welcome the new members who joined in April, May and June of 2020!
Pharmacy Action Fund Contributors
Thank you to everyone who's donated to the ICHP PAC over the past year!
ICHP Board of Directors 2020 - 2021
Board of Directors
|
Carrie Vogler
President
|
Julie Downen
Regional Director
Central
|
David Martin
Educational Affairs
Director
|
Tara Vickery Gorden
Small and Rural Hospital
Network Chair
|
|
Noelle Chapman
Immediate Past
President
|
Alifiya Hyderi
Regional Director
Northern
|
Bernice Man
Marketing Affairs
Director
|
David Tjhio
Committee on
Technology Chair
|
|
Jen Arnoldi
President-Elect
|
Jared Sheley
Regional Director
Southern
|
Sharon Karina
Government Affairs
Director
|
Jennier Phillips
Editor & Chair,
KeePosted
|
|
Christopher Crank
Treasurer
|
Kristine VanKuiken
Technician
Representative
|
Natalie Tucker
New Practitioners
Network Chair
|
Milena Murray
Assistant Editor,
KeePosted
|
|
Ed Rainville
Secretary
|
Elise Wozniak
Organizational Affairs
Director
|
Dan Majerczyk
Ambulatory Care
Network Chair
|
Nora Flint
Residency Leaders
Network Chair
|
|
Scott Meyers
Executive Vice
President
ICHP Office
|
Amy Boblitt
Professional Affairs
Director
|
Becky Ohrmund
Pharmacy Technician
Network Chair
|
|
Student Society Presidents
Sanad Abduljawad
Chicago State University College of Pharmacy
|
Kristen Ingold
Southern Illinois University Edwardsville
School of Pharmacy
|
Irum Khan
Midwestern University Chicago
College of Pharmacy
|
Josiah Baker
University of Illinois at Chicago
College of Pharmacy
|
Jeremy Fernandez Balingit
Roosevelt University College of Pharmacy
|
Bill Clafshenkel
University of Illinois at Chicago
Rockford Campus College of Pharmacy
|
Nimita Shah
Rosalind Franklin University
College of Pharmacy
|
|
Northern Illinois Society of Health-System Pharmacists (NISHP)
Milena Murray
President
|
Andrew Merker
President-Elect
|
Denise Kolanczyk
Immediate Past President
|
Erin Shaughnessy
Treasurer
|
David Martin
Secretary
|
Richard Puccetti
Technician Representative
|
West Central Society of Health System Pharmacists
Liz Harthan
President
|
Ed Rainville
Immediate Past-President
|
Metro East Society of Health-System Pharmacists (MESHP)
Sangamiss Society of Health-System Pharmacists
Megan Stoller
President
|
Ashlie Kallal
President-Elect
|
Billee Samples
Immediate Past-President
|
Vacant Roles at Affiliates
President, Rock Valley Society
President, Southern IL Society
President, Sugar Creek Society
Print Entire Issue


 President's Message
President's Message Directly Speaking
Directly Speaking