Official Newsjournal of the Illinois Council of Health-System Pharmacists

November 2021
Volume 47 Issue 4
Columns
Features
2020 Spring Meeting Poster Presentations
Opioid Task Force - CPE Opportunity!
College Connection
Midwestern University Chicago College of Pharmacy
Roosevelt University College of Pharmacy
Rosalind Franklin University of Medicine and Science College of Pharmacy
Southern Illinois University Edwardsville (SIUE) - School of Pharmacy
University of Illinois at Chicago College of Pharmacy
More
ICHP Pharmacy Action Fund (PAC)
Board of Directors, Student Society Presidents & Affiliates
ICHP Info

Illinois Council of Health-System Pharmacists
4055 North Perryville Road
Loves Park, IL 61111-8653
Phone: (815) 227-9292
Fax: (815) 227-9294
ichpnet.org
KeePosted
Official News journal of the Illinois Council of Health-System Pharmacists
EDITOR
Jennifer Phillips
ASSISTANT EDITOR
Milena Murray
MANAGING EDITOR
Scott Meyers
ASSISTANT MANAGING EDITOR
Trish Wegner
DESIGN EDITOR
Melissa Dyrdahl
ICHP Staff
EXECUTIVE VICE PRESIDENT
Scott Meyers
VICE PRESIDENT - PROFESSIONAL SERVICES
Trish Wegner
DIRECTOR OF OPERATIONS
Maggie Allen
INFORMATION SPECIALIST
Heidi Sunday
CUSTOMER SERVICE AND
PHARMACY TECH TOPICS™ SPECIALIST
Jo Ann Haley
ACCOUNTANT
Jan Mark
COMMUNICATIONS MANAGER
Melissa Dyrdahl
LEGISLATIVE CONSULTANTS
Liz Brown Reeves
Mitch Schaben
ICHP's Mission Statement
Advancing Excellence in Pharmacy
ICHP's Vision Statement
ICHP dedicates itself to achieving a vision of pharmacy practice where:
· Pharmacists are universally recognized as health care professionals and essential providers of health care services.
· Pharmacists use their medication expertise and leadership skills to optimize the medication use process and patient outcomes.
· Pharmacy technicians are trained and PTCB certified to manage the medication distribution process.
ICHP's Goal Statements
· Raising awareness of the critical role pharmacists fulfill in optimizing medication therapy and ensuring medication safety in team-based, patient-centered care.
· Providing high quality educational services through innovative continuing pharmacy education and training programs, and sharing evidence-based best practices.
· Developing and nurturing leaders through mentorship, skill development programs, and leadership opportunities.
· Working with national and state legislators and policymakers to create or revise legislation and regulation critical to pharmacy practice and quality patient care.
· Urging pharmacy technician employers to require successful completion of an accredited pharmacy technician training program and PTCB certification of all pharmacy technicians.
Approved by the ICHP Board of Directors May 30, 2018.
KeePosted Vision
As an integral publication of the Illinois Council of Health-System
Pharmacists, the KeePosted newsjournal will reflect its
mission and goals. In conjunction with those goals, KeePosted will
provide timely information that meets the changing professional and personal
needs of Illinois pharmacists and technicians, and maintain high publication
standards.
KeePosted is an official publication of, and is copyrighted by, the
Illinois Council of Health-System Pharmacists (ICHP). KeePosted is
published 4 times a year. ICHP members received KeePosted as
a member benefit. All articles published herein represent the opinions of the
authors and do not reflect the policy of the ICHP or the authors’ institutions
unless specified. Advertising inquiries can be directed to ICHP office at the
address listed above. Image disclaimer: The image used in the Pharmacy Tech
Topics™ advertisement is the property of © 2017 Thinkstock, a division of Getty
Images. Some images are property of © 2020 Adobe Stock.
Copyright © 2020, Illinois Council of Health-System Pharmacists. All rights
reserved.
Columns
 President's Message
President's Message
La Farmacia Esta Abierta
by Carrie Vogler, PharmD, BCPS, Clinical Associate Professor - SIUE School of Pharmacy
give back. ICHP provides education to you so you can better care for others. I encourage you to spread kindness to those less fortunate by sharing your knowledge and time with your patients.

 Directly Speaking
Directly Speaking
Dealing with COVID-19!
by Scott A. Meyers, Executive Vice President
While planning for disaster has been in place for many years, we are still poorly resourced for a pandemic or disaster of this nature! While good business practices in the commercial world tout “just in time” inventory, healthcare needs a bigger stockpile or better controls of the inventory when we see disaster approaching. Hoarding by consumers, individual health-systems, and even physicians of potential medication options is disgraceful and unethical. We need to identify triggers or alerts that can initiate immediate holds on vital supplies. And, as individuals, we need to make sure the pantry is filled a little better just in case that disaster hits and the holds are initiated in a timelier manner. My goodness, just $10 more a pay period and before you know it, you’ll have a year’s supply of toilet paper in your linen closet. Then, like the good inventory control person that pharmacists are, you rotate your stock and all’s well.
Finally, and this one is the toughest, maybe we should try to convince our pharmaceutical manufacturers to produce more of their medications right here in the good old USA. While we are a part of a global economy, being dependent on foreign manufacturers for raw materials and even a large portion of our generic medication supply is proving to be problematic. Even before CV-19! Yes, we will most likely have to pay more for these products, but the supply chain will be more stable and the impact of disaster half-way around the world will have a smaller ripple when it hits here. Not to mention, our nation could get back into the business of helping other countries out when the feces hits the fan somewhere else.
It's Time To Step Up
The Nominations Committee is Calling!
by Scott A. Meyers, Executive Vice President
ICHP Best Practice Award
- Innovativeness / originality
- Contribution to improving patient care
- Contribution to institution and pharmacy practice
- Scope of project
- Quality of submission
- Introduction, Purpose, and Goals of the program
- Description of the program
- Experience with and outcomes of the program
- Discussion of innovative aspects of programs and achievement of goals
- Conclusion
- The program will be featured at the ICHP Annual Meeting. You will need to prepare a poster to present your program and/or give a verbal presentation. Guidelines will be sent to the winner.
- You may be asked to electronically submit your manuscript to the ICHP KeePosted™ for publishing as a continuing pharmacy education home study program.
- You will receive a complimentary registration to the ICHP Annual Meeting, recognition at the meeting and a monetary award distributed to your institution.
Recognize the Best
ICHP Awards Process Opens
by Scott A. Meyers, Executive Vice President
- The nominee is a person of high moral character, good citizenship and high professional ideals;
- The nominee has made significant contributions affecting the practice of health-system pharmacy throughout the State; and
- These contributions should be in the form of sustained exemplary service in health-system pharmacy or a single outstanding achievement, or a combination of accomplishments benefiting health-system pharmacy, through it, humanity, and the public health.
- Health-system pharmacy practice
- Health-system pharmacy education
- Health-system pharmacy administration
- Pharmaceutical research or development related to health-system pharmacy
- Pharmacy organizational activity with a definite relationship to health-system pharmacy
- Scientific or professional pharmacy writing, (e.g., noteworthy articles on pharmaceutical subjects with applicability to health-system pharmacy)
- Pharmaceutical journalism related to health-system pharmacy
- Public and/or inter-professional relations activities benefiting health-system pharmacy
- Pharmacy law or legislation, professional regulations, standards of professional conduct or pharmacy law enforcement as related to health-system pharmacy.
- The individual nominated to receive this award must be an ICHP pharmacist, associate or technician member in good standing;
- The individual should be an exemplary preceptor, professor and/or mentor of students, residents, pharmacy technicians and/or new practitioners;
- The individual should be a positive role model for pharmacists, pharmacy students and/or pharmacy technicians;
- Please provide your name(s), i.e., the name of the nominator(s). (More than one person can nominate a nominee).
- Provide the name of the person you are nominating. In addition, the nominee’s curriculum vitae must be included in the nomination package.
- Is the nominee a member of ICHP?
- In what capacity have you worked with the nominee?
- In what ways do you see the nominee working to advance the profession of pharmacy?
- Give some examples of ways in which this nominee is a model mentor/preceptor.
- Give some examples in which this nominee has demonstrated a service to community (outside of job responsibilities).
- How has this nominee impacted your career?
- Be a current ICHP technician member,
- Be a PTCB certified pharmacy technician for at least two years,
- Demonstrate at least one of the following:
- exceptional contributions to his/her pharmacy worksite
- exceptional contributions to ICHP as a volunteer member
- exceptional contributions to the practice of pharmacy in Illinois
- Be the technician’s supervisor, colleague, or co-worker. No self-nominations will be accepted.
- Worksite name, address and telephone number.
- Technician name and year certified.
- Describe the technician’s contributions in detail.
- Deadline for nominations is July 1, 2020.
- All nominations are reviewed by the Division of Marketing Affairs at their July conference call.
- The Division will select two finalists for consideration and present them to the ICHP Board of Directors at the July Board Meeting.
- The ICHP Board of Directors will select the award winner from the two finalists presented by the Division of Marketing Affairs.
- The Division of Marketing Affairs may recommend one of the two finalists by providing detailed discussion points to the Board of Directors.
- The Board is not required to select a recipient if no candidate seems qualified.
- The Award recipient and her/his nominator will be notified immediately following his/her selection by the Board of Directors.
- The award recipient will receive a complimentary full registration to the ICHP Annual Meeting.
- The award recipient will receive a plaque to be presented at an appropriately agreed upon time during the ICHP Annual Meeting.
Board of Pharmacy Update
Highlights of the January 2020 Meeting
by Scott A. Meyers, Executive Vice President
- March 10th - Chicago
- May 12th - Springfield*
- July 14th - Chicago
- September 8th - Chicago
- November 10th - Chicago
- Who needs to obtain the Sexual Harassment Prevention CE before license renewal in March?
Pharmacists and Certified Pharmacy Technician will need to obtain the credit while students, grandfathered and new technicians will not. Student pharmacists who are certified pharmacy technicians will need to obtain the CE credit if they wish to maintain their certified pharmacy technician status on their Illinois license. - Will the Department investigators be enforcing the record keeping requirements for meal and rest breaks right away?
The Department intends to spend the first three months of 2020 educating pharmacists-in-charge on how to record breaks. Only breaks for pharmacists must be recorded. Pharmacists-in-charge should begin working on this requirement immediately. - Do the new revisions to the Act now officially allow pharmacy technicians to administer immunizations?
Pharmacy technicians and certified pharmacy technicians may administer immunizations if they have been trained using a Department approved training program as long as the pharmacist on duty has reviewed the patient profile to determine appropriateness. To date no training programs have been reviewed.
Board of Pharmacy Update
Highlights of the March 2020 Meeting
by Scott A. Meyers, Executive Vice President
ICHPeople
 Desi Kotis
Desi KotisCongratulations and sad farewell to Dr. Desi Kotis, Director of Pharmacy at Northwestern Memorial Hospital, who is leaving her position to take a bigger one at the University of California San Francisco Health. Desi will serve as Chief Pharmacy Officer for the entire UCSF Health system, overseeing hospitals, clinics and outpatient pharmacy services. In addition, Desi will step down from her appointment to the Board of Pharmacy. She will maintain a residence in Illinois in addition to one in the Bay area, so don’t be surprised if she pops into an ICHP statewide meeting in the future. Plus we know we’ll see her at ASHP meetings around the country. Congratulations Desi, we’ll miss you!
Congratulations to Dr. Jason Alegro at the Roosevelt University College of Pharmacy (RUCOP)! RUCOP was selected as a site for the Midwest integration of the National HIV Curriculum Project. Dr. Alegro, an Assistant Professor at RUCOP, will lead the incorporation of the project into coursework which will provide students with the most up-to-date HIV/AIDS training.
Julia Helvi Hamrén was born 3/3/2020. She came into the world weighing 6 lbs 14 ounces and was 20 inches long. She was welcomed by proud parents, Rachael Prusi and Jonas Hamrén. Welcome Baby Julia!
Email melissad@ichpnet.org to be featured in our next issue of KeePosted.

Government Affairs
The Bills, Task Force and COVID-19
by Scott A. Meyers, Executive Vice President
- SB2972 - Allows pharmacists to dispense hormonal contraceptives by standing order.
- SB3147 - Allows pharmacists to dispense smoking cessation products by standing order (including prescription products).
- SB3266 - Would require hospitals and surgery centers to offer to provide multi-dose medications to patients upon discharge with proper outpatient labeling.
- HB4362 - Creates the Wholesale Importation of Prescription Drugs Act and allows importation from Canada.
- HB4475 - Changes the decision to declare an emergency with regard to the 12-hour shift length limit from the pharmacist to the PIC. This bill may also be a vehicle for other changes related to the new “Pharmacy Working Conditions” section of the Practice Act (meal and rest break records and resident shift length).
- HB5659 - Creates a Disciplinary Review Board to oversee pharmacy-related disciplines by the Department.
Reconfirmed support for implementation of a CQI program for every pharmacy with all internal documents protected for discovery for criminal or civil purposes.
Establishment of a new Task Force focused on new and more appropriate reimbursement models for pharmacist-provided patient care services.
- Point of care testing
- Standardized standing order processes from IDPH
- Medication administration by pharmacists
- Review of longevity of prescription refills
- Methods for better enforcement of existing pharmacy regulations
- CDC COVID-19 website
- ASHP COVID-19 resource page
- Multiple documents from State of Illinois Agencies and IPhA
- Updates from USP
New Practitioners Network
by Natalie R. Tucker, PharmD, BCPS, BCIDP; Chair, New Practitioners Network; Clinical Pharmacy Specialist, Antimicrobial Stewardship, HSHS St. John's Hospital
- Obtaining medications
- Storing, labeling, and dispensing medications
- Developing guidelines for the diagnosis and treatment of affected patients
- Consulting with providers on treatment for individual patients
- Advising the public on the appropriate use of medications in response to disasters
These recommendations may seem like part of your normal daily tasks, and in fact they are! One key fact to remember is that we already have the necessary skills to perform these duties. During pharmacy school, residency training, and in our daily work, we use these skills every day whether we realize it or not.
- Educate yourself on your local and institutional plans for emergency preparedness
- Share evidence-based recommendations for treatments with colleagues
- Act assertively to prevent panic and irrational responses to disasters
- Obtain basic cardiac life support (BCLS) certification through the American Heart Association
- Obtain Pharmacy-Based Immunization Delivery certification through the American Pharmacists Association
- American Society of Health-System Pharmacists. ASHP statement on the role of health-system pharmacists in emergency preparedness. Am J Health-Syst Pharm. 2003;60:1993-1995.
- Narayann N, Lacy CR, Cruz JE, et al. Disaster preparedness: biological threats and treatment options. Pharmacotherapy. 2018;38(2):217-234.
Residency Leaders Network
Call for Volunteers
by Nora B. Flint, PharmD, FASHP, BCPS; Chair, ICHP Residency Leaders Network; Associate Corporate Director; Director, PGY1 Residency Program, Department of Pharmacy - Rush University Medical Center
The mission of the ICHP RLN is to offer networking opportunities to resolve questions, challenges, and issues that residency directors and residency coordinators often face when guiding residents through their program and/or preparing for ASHP accreditation.
If you are interested in joining this new network, please contact Trish Wegner at TrishW@ichpnet.org.
Hi Tech
New Technician Network - 1st Call
by Becky Ohrmund, CPhT, Pharmacy Technician Specialist, Pharmacy Department, Northwestern Memorial Hospital
ICHP Leadership Spotlight
Liz Harthan, PharmD, BCPS
 What is your leadership position in ICHP?
What is your leadership position in ICHP?I am the Anticoagulation Pharmacotherapist Coordinator at OSF Healthcare. The easiest way to describe my job is a medication safety pharmacist with a single focus on anticoagulants. My goal is to ensure our pharmacists and other healthcare providers have the tools and knowledge they need to use anticoagulants safely.
Prescription drug pricing and medication shortages are something that has affected my professional life, as it has all pharmacists, but they have also affected my family. My mom has Wilson’s disease and has been taking penicillamine for over 40 years. Prior to about 8 years ago, this orphan drug cost around $350 per month. Her insurance company treated this as a tier 3 medication and she had a very reasonable co-pay. This was one of those drugs that saw a 2700% price increase almost overnight for no apparent reason other than drug company profit. My mom’s reasonable co-pay became completely unaffordable. Additionally, this drug began showing up on and off the shortage list. Luckily, she is a well-educated person with a pharmacist for a daughter. We have been able to jump through the correct hoops, fill out loads of paperwork, contact pharmacy suppliers, and mostly keep her with a regular supply of medication. I am absolutely certain there are patients who require this drug that are unable to maneuver the unreasonable cost increases and drug shortages, and have had to go without treatment. I worry about my mom and the other patients who have been negatively affected by corporate greed.
One of the particular challenges I faced was helping our health system determine the best formulary choice for the oral Factor Xa inhibitor reversal. On the surface, this would seem like an easy choice. Choose an FDA approved reversal agent or continue using a product off-label. Unfortunately, the choice was not that straightforward. Extreme drug costs, challenging pharmacokinetics, and a complex patient population made the decision all the more challenging. In the end, I believe we made the best choice for our health system by completing a thorough evaluation of safety, efficacy, and cost and selected the non-FDA indicated drug. However, I can say it was the most difficult formulary decision of which I have been a part.
ICHP is great because it provides an easy way for you to get involved. There are so many opportunities to take a leadership role without feeling like you have committed all of your free time. It’s really easy to start off simply by becoming a site champion. From there you can try out new roles and opportunities.
My co-workers! I saw many of my co-workers getting involved in ICHP, networking with other pharmacists across the state, and gaining leadership experience. I was almost jealous, but realized it was easy for me to be able to do the same.
Pharmacy is such a small world. The bridges you build or burn as a student will impact the opportunities you seek, as you move forward in your career. As the job field becomes more competitive, use your P4 year as a spring board for beginning a successful profession. Before picking rotations, do your homework. What areas of pharmacy do you want to explore? What would truly be a good fit for you? Don’t just pick the rotation that sounds exciting. Pick the one that will give insight into your future job or help you take the next steps. If you want to work in a hospital, check out different settings. Do you like the atmosphere of critical care or critical access? Are you interested in residency? Try to select rotations where you would consider applying for residency or where there are residents so you can ask for their advice. There are so many opportunities in pharmacy, but make your years as a student count!
I really enjoy cooking (not to be confused with baking). However, with two small children, I don’t get to be as adventurous in my recipe selection as I once was. I do still enjoy trying new things and cooking for my family.
I really love pizza, but I’m a total pizza snob so I rarely eat it. I grew up in the Chicago suburbs eating real pizza. :)
Professional Affairs
Pharmacist-Led Implementation of a Direct Oral Anticoagulant Prescribing Guideline and Evaluation of Prescribing Practices at a Community Teaching Hospital
by Paula Bielnicka, PharmD; Clinical Staff Pharmacist & Outpatient Anticoagulation Pharmacist, Swedish Hospital, Part of NorthShore, Chicago, IL and Alicia Juska, PharmD, BCPS; Director of Pharmacy Services/Residency Program Director, Swedish Hospital, Part of NorthShore, Chicago, IL and John Shilka, PharmD, BCPS; Clinical Pharmacist, Managed Care/Internal Medicine, University of Illinois at Chicago - College of Pharmacy, Chicago, IL
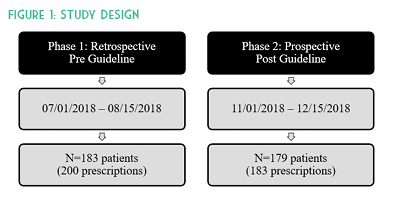
Creation of a hospital formulary-specific DOAC guideline standardizing rivaroxaban and apixaban doses per anticoagulation indication and duration was approved by the Pharmacy & Therapeutics Committee and the project was reviewed by an Institutional Review Board. Outpatient prescriptions were not evaluated. Patients were eligible for inclusion in this study if they were treated with apixaban or rivaroxaban, were at least 18 years of age, and were not pregnant. The primary outcome measured the rate of appropriate inpatient prescriptions concordant with package insert labeling. Inappropriate prescriptions were classified as either an overdose or under-dose to determine where healthcare provider education was needed. Secondary outcomes included number of thrombotic or bleeding events during hospitalization, readmissions for bleeds/thromboembolisms, and patients with end stage renal disease.
A total of 383 orders were included with 200 prescriptions in the pre-guideline arm and 183 prescriptions in the post-guideline arm. Baseline characteristics were similar in age, anticoagulation indication, and about half of all patients in each group had a creatinine clearance of 15 to 50 mL/min. Overall there were more males in the pre-guideline arm (64% versus 46%) and approximately 24% of all patients were above100 kg. No p-values were calculated for baseline characteristics (See Table 1). DOACs were appropriately dosed in 87% (175/200) versus 93% (171/183) of prescriptions pre- and post-guideline implementation, respectively. Evaluation of inappropriate apixaban prescriptions alone identified 16 (10%) pre-guideline compared with 10 (7%) post-guideline. Assessment of rivaroxaban dosing alone identified nine (27%) inappropriate pre-guideline doses compared to two (5%) post-guideline doses. After investigating the 37 inappropriate hospital DOAC prescriptions, it was determined that more than half were due to continuation of a patient’s original home dose upon hospital admission. Missed opportunities were due to both pharmacist and provider hesitation to change established home dosages and from providers denying auto substitutions. (See Table 2.)
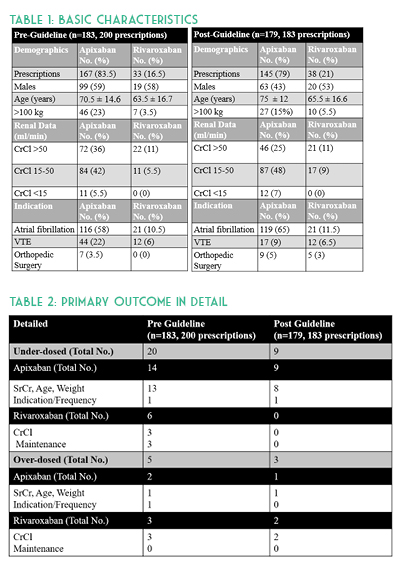
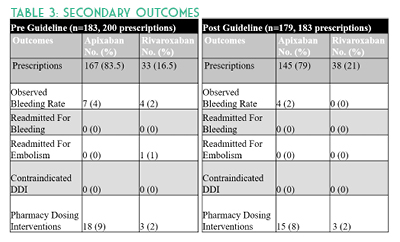
Study strengths included use of an electronic medical record (EMR) system which contains medication detail, including patients’ external fill history, patient specific information, prescriber documentation, and laboratory results. This allowed for more accurate assessment of dosing appropriateness and potential for drug-drug interactions. Implementation of pharmacist-led renal dose adjustments allowed for timely administration of appropriate doses based on daily hospital laboratory results. Study limitations include a small sample size and timeframe compared to previously published DOAC studies.4-6 Some patient information was missing at time of inpatient order verification, which was later discovered during the visit. For instance, anticoagulation indication and appropriateness were unclear for AF patients hospitalized for another indication such as DVT. These occurrences were not excluded, but rather the newest diagnosis was evaluated as the primary anticoagulation indication in the retrospective data collection. Another limitation is that CHA2DS2-VASc scores were not calculated and therefore not used to determine anticoagulation appropriateness in AF patients. Discharge prescriptions were not evaluated in this study. Other barriers include potential drug-drug interactions due to unavailable external medication fill histories and incomplete medication histories. Future studies should evaluate the home DOAC regimen and appropriateness of continuing this on hospital admission as well as upon discharge.
Results suggest underdosing in apixaban patients, especially continuation of home doses upon admission, may require further provider education. As evidenced by this study, implementation of a pharmacist-led DOAC guideline allowing for renal dose adjustments may improve both FDA-approved concordant prescribing of these medications and decrease rates of bleeding.
- Eliquis (apixaban) package insert. Princeton, NJ: Bristol-Myers Squibb Company and Pfizer Inc.; 2015.
- Xarelto (rivaroxaban) package insert. Titusville, NJ: Janssen Pharmaceuticals, Inc.; 2012.
- Yao X, Shah ND, Sangaralingham LR, Gersh BJ, Noseworthy PA. Non-vitamin k antagonist oral anticoagulant dosing in patients with atrial fibrillation and renal dysfunction. J Am Coll Cardiol. 2017;69(23):2779-2790.
- Ruiz OM, Muniz J, Rana M, et al. Inappropriate doses of direct oral anticoagulants in real-world clinical practice: prevalence and associated factors. A subanalysis of the FANTASSIA Registry. Europace. 2018;20(10):1577-1583.
- McAlister FA, Garrison S, Kosowan L, Ezekowitz JA, Singer A. Use of direct oral anticoagulants in Canadian primary care practice 2010–2015: A cohort study from the Canadian primary care sentinel surveillance network. J Am Heart Assoc. 2018;7(3): e007603. https://www.ahajournals.org/doi/full/10.1161/JAHA.117.007603. (accessed 2018 May 27).
- Steinberg BA, Shrader P, Thomas L, et al. Off-label dosing of non-vitamin k antagonist oral anticoagulants and adverse outcomes: the ORBIT-AF II Registry. J Am Coll Cardiol. 2016;68(24):2597-2604.
Educational Affairs
ASA for Primary Prevention - A Worthy Topic for Shared-Decision Making
by By Regina Arellano, Pharm.D., BCPS Assistant Professor Midwestern University Chicago College of Pharmacy and Jaini Patel, Pharm.D., BCACP Assistant Professor Midwestern University Chicago College of Pharmacy
- Miner J, Hoffhines A. The discovery of aspirin’s antithrombotic effects. Tex Heart Inst J 2007;34:179–186.
- Antithrombotic Trialists' Collaboration. Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ 2002;324:71.
- Selak V, Kerr A, Poppe K, et al. Annual Risk of Major Bleeding Among Persons Without Cardiovascular Disease Not Receiving Antiplatelet Therapy. JAMA 2018; 319:2507.
- Whitlock EP, Burda BU, Williams SB, et al. Bleeding Risks With Aspirin Use for Primary Prevention in Adults: A Systematic Review for the U.S. Preventive Services Task Force. Ann Intern Med 2016; 164:826.
- Miedema MD, Huguelet J, Virani SS. Aspirin for the primary prevention of cardiovascular disease: in need of clarity. Curr Atheroscler Rep 2016;18:4.
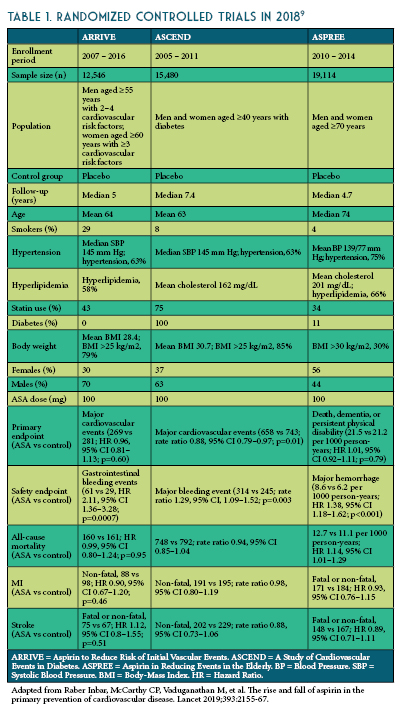
- Gaziano JM, Brotons C, Coppolecchia R, et al. Use of aspirin to reduce risk of initial vascular events in patients at moderate risk of cardiovascular disease (ARRIVE): a randomised, double-blind, placebo-controlled trial. Lancet 2018; 392:1036.
- ASCEND Study Collaborative Group. Effects of aspirin for primary prevention in persons with diabetes mellitus. N Engl J Med 2018;379:1529.
- ASPREE
- Raber Inbar, McCarthy CP, Vaduganathan M, et al. The rise and fall of aspirin in the primary prevention of cardiovascular disease. Lancet 2019;393:2155-67.
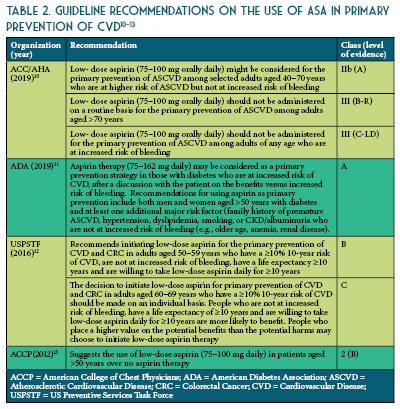
- Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease. JACC 2019;74:e177-232.
- ADA. Cardiovascular disease and risk management. Diabetes Care 2019;42(1):103.
- Bibbins-Domingo, K, on behalf of the U.S. Preventative Services Task Force. Aspirin use for the primary prevention of cardiovascular disease and colorectal cancer: U.S. Preventative Services Task Force Recommendation Statement. Ann Intern Med 2016;164:836-845.
- Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012;141:e637S-e668S.
- Abdelaziz HK, Saad M, Pothineni N, et al. Aspirin for primary prevention of cardiovascular events. JACC 2019;73:2915-2929.
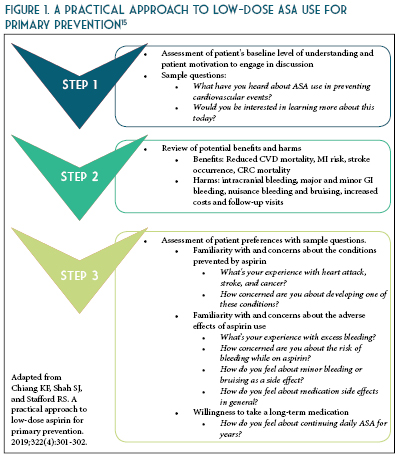
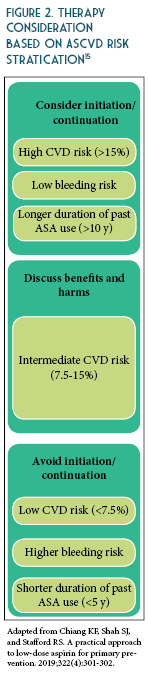
- Chiang KF, Shah SJ, and Stafford RS. A practical approach to low-dose aspirin for primary prevention. JAMA 2019;322(4):301-302.
Features
2020 Spring Meeting Poster Presentations
KeePosted Op Ed
An Introduction to Single Payer for Pharmacists and Pharmacy Technicians
Feature Article
by Shannon M. Rotolo, PharmD, BCPS; Clinical Pharmacy Specialist; U Chicago Medicine and Randall W. Knoebel, PharmD, BCOP; Senior Manager, PHarmacy Health Analytics, Drug Policy & High Reliability; UChicago Medicine
“Opinions expressed by authors of Op-Ed articles in the KeePosted are their own and are not necessarily shared by ICHP or its members. ICHP will publish these articles from time to time to direct you to topics that may be of interest to you and to stimulate discussion on potentially controversial issues of the day.”
What is single-payer?
Single-payer national health insurance describes a system in which one public agency is responsible for health care coverage, but the delivery of health care services continue to be provided by mostly private businesses.1 This means all payments health care services would come from one agency, but physicians’ offices, hospitals, and pharmacies would continue to be owned and operated by organizations or individuals, as they are in the current system. The two proposed bills for single-payer in the United States are commonly known as Medicare for All. While there are differences between the House bill2 and the Senate bill3, both endeavor to provide robust health care coverage – medical, pharmacy, dental, vision, etc. – for everyone living in the US and to eliminate the private insurance industry’s role in covering health care services included in this legislation. Projected costs vary between analyses4, but most show the overall cost of health care services remaining about the same or decreasing, due to minor increases in utilization as more uninsured or underinsured people can afford to seek the care they need, coupled with major decreases in overhead – Medicare spends about 2% on administrative costs compared to private insurers spending up to 18%5 – and decreased cost of health care services, including drug pricing.
Perhaps the most obvious change that would impact pharmacy is in drug pricing. Brand name drug prices have increased dramatically in the last decade.6 Based on data from Australia and New Zealand, some experts estimate the cost of brand name drugs would drop as much as 50%. While the generic market is less likely to see dramatic price changes, there is greater opportunity for rapid intervention and improved access during drug shortages and for negotiation on prices with both brand and generic manufacturers of “me too” drugs if there is one national formulary.7 This is a role some argue pharmacy benefit managers (PBMs) can play, but again, drug prices continue to climb. In scenarios where savings are achieved by a PBM this is often to the benefit of their shareholders or partnering businesses, rather than to taxpayers or patients.8 By consolidating negotiating power with a single-payer, there would be greater leverage over drug companies’ asking prices.
Currently proposed Medicare for All bills in the House and Senate differ in their plans for prescription coverage. The House bill calls for no deductibles and no copays at any point. The Senate bill would allow for up to $200 per year in out-of-pocket prescription costs. This may sound like a small distinction, but has the potential to disproportionately impact low income patients.10 Pharmacists who have worked with patients with high-deductible prescription plans will immediately recognize this issue. Physicians may not, but they are the primary health care professionals advocating for Medicare for All.11 Most physicians have a limited understanding of the intricacies of pharmacy billing and reimbursement.12 We don’t know if single-payer will move forward in the next few years, or if it will take several decades, but we do know pharmacists will need to have a seat at the table when the time comes to ensure the changes made are appropriate and sustainable. The best way to guarantee that seat at the table is to get involved in the conversations happening around single-payer now.
To our knowledge, there are less than a dozen pharmacists actively involved in the single-payer movement right now, as compared to the over 23,000 physicians and 1,200 medical students who are members of Physicians for a National Health Plan (PNHP). We know pharmacists are extraordinarily effective advocates when we work for change to improve the lives of our patients and to advance our profession.13 We continue to rank among the top professions year after year for honesty and ethical standards.14 We are trusted experts, and we have the authority to speak on issues facing our broken health care system, particularly when it comes to medications. It’s time to put our expertise to use in advocating for structural change, and to make sure the plans behind it support our patients and align with our goals as a profession.
For those looking to get involved, two Illinois based organizations that focus on this issue are IL Single Payer Coalition (http://ilsinglepayer.org/) and PNHP Illinois (https://pnhp.org/chapter/illinois/). Other ways to take action include contacting your state and federal legislators to ask them to support single payer legislation, writing op-eds or letters to the editor in local newspapers, public speaking, lobbying, and organizing.
- Physicians for a National Health Program. About Single Payer. https://pnhp.org/what-is-single-payer/ (accessed 2019 Nov 22).
- Medicare for All Act of 2019, H.R.1384, 116th Cong. (2019). https://www.congress.gov/bill/116th-congress/house-bill/1384/text (accessed 2019 Nov 22).
- Medicare for All Act of 2019, D.1129, 116th Cong. (2019). https://www.congress.gov/bill/116th-congress/senate-bill/1129/text (accessed 2019 Nov 22).
- The New York Times. Would ‘Medicare for All’ Save Billions or Cost Billions? https://www.nytimes.com/interactive/2019/04/10/upshot/medicare-for-all-bernie-sanders-cost-estimates.html (accessed 2019 Nov 22).
- Center for Economic and Policy Research. Overhead Costs for Private Health Insurance Keep Rising, Even as Costs Fall for Other Types of Insurance. http://cepr.net/blogs/cepr-blog/overhead-costs-for-private-health-insurance-keep-rising-even-as-costs-fall-for-other-types-of-insurance (accessed 2019 Nov 22).
- Wineinger NE, Zhang Y, Topol EJ. Trends in Prices of Popular Brand-Name Prescription Drugs in the United States. JAMA Netw Open. 2019 May 3;2(5):e194791.
- Gaffney A, Lexchin J; US; Canadian Pharmaceutical Policy Reform Working Group. Healing an ailing pharmaceutical system: prescription for reform for United States and Canada. BMJ. 2018 May 17;361:k1039.
- The Commonwealth Fund. Pharmacy Benefit Managers and Their Role in Drug Spending. https://www.commonwealthfund.org/publications/explainer/2019/apr/pharmacy-benefit-managers-and-their-role-drug-spending (accessed 2019 Nov 22).
- Heidari P, Cross W, Weller C, Nazarinia M, Crawford K. Medication adherence and cost-related medication non-adherence in patients with rheumatoid arthritis: A cross-sectional study. Int J Rheum Dis. 2019 Apr;22(4):555-566.
- Kaiser Family Fund. Medicaid. The Effects of Premiums and Cost Sharing on Low-Income Populations: Updated Review of Research Findings. https://www.kff.org/medicaid/issue-brief/the-effects-of-premiums-and-cost-sharing-on-low-income-populations-updated-review-of-research-findings/ (accessed 2019 Nov 22).
- TIME. A New Generation of Activist Doctors Is Fighting for Medicare for All. https://time.com/5709017/medicare-for-all-doctor-activists/ (accessed 2019 Nov 22).
- Tseng, C., Lin, G.A., Davis, J. et al. Giving formulary and drug cost information to providers and impact on medication cost and use: a longitudinal non-randomized study. BMC Health Serv Res. 2016 Sep 21;16(1):499.
- Little J, Ortega M, Powell M, Hamm M. ASHP Statement on Advocacy as a Professional Obligation. Am J Health Syst Pharm. 2019 Feb 1;76(4):251-253.
- Forbes. America's Most & Least Trusted Professions. https://www.forbes.com/sites/niallmccarthy/2019/01/11/americas-most-least-trusted-professions-infographic/#43777eba7e94 (accessed 2019 Nov 22).
Opioid Task Force - CPE Opportunity!
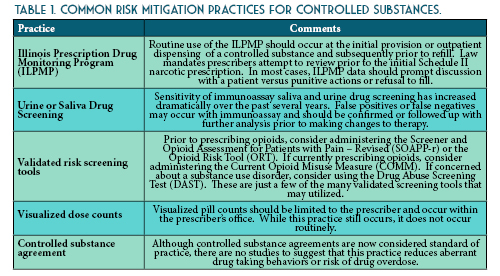
Common Questions about the Illinois Prescription Drug Monitoring Program
Feature Article
by Chris Herndon, PharmD, BCACP; Professor, School of Pharmacy, Southern Illinois University Edwardsville, Edwardsville, IL and Sarah Pointer, PharmD; Clinical Director of the Prescription Monitoring Program, Bureau of Pharmacy and Clinical Support Services, Springfield, IL
How has the ILPMP impacted opioid overdose rates in Illinois?
As the million-dollar question, the impact of the ILPMP on opioid mortality in Illinois may be the most difficult question to answer. Several studies have shown a direct correlation between the enactment of prescription drug monitoring program laws and opioid prescribing rates.1–3 Based on the Centers for Disease Control and Prevention (CDC) 2019 Surveillance Report of Drug-Related Risks and Outcomes, Illinois has among the lowest rates of long-acting or extended release opioid prescriptions and among the lowest rate of high-dosage opioid prescribing (defined as morphine milligram equivalents greater than 90 mg daily).4 Unfortunately, Illinois ranks 14th in the nation for age-adjusted drug overdose deaths per 100,000 population. While the ILPMP and other risk mitigation practices has undoubtedly reduced prescription substance abuse and diversion, a swift and unprecedented shift to synthetic and semi-synthetic illicit opioids has driven the steady increase in mortality rates. However, in 2018, Illinois realized its first decrease in opioid mortality rate with a lookback period of 5 years.
Access to prescription drug monitoring program data has long been a topic of great contention. The earliest state drug monitoring programs were housed within various agencies of law enforcement (e.g. California & Hawaii). The Illinois prescription monitoring program was the first to be housed within a state Department of Health. This had significant ramifications on the privacy of personal health data and the access to such data by law enforcement. In Illinois, law enforcement may only request indirect access for active cases under investigation.
Utilizing a patient’s first name, last name, and date of birth is a good place to start for most searches. However, the database is only as accurate as the prescription information uploaded to the system. For instance, if you were to search Christopher Herndon, you may miss data that was uploaded as Chris Herndon. For this reason, I usually recommend using the first four letters of the last name and the first three letters of the first name to reduce the risk of missing results due to variations in spelling of the name. The ILPMP also allows users to simultaneously search other state monitoring programs. Illinois currently has agreements to share data with 22 other states, including all bordering states (and Missouri’s St. Louis County PDMP).
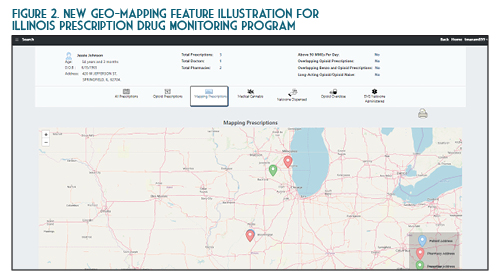
Once you verify your search results, the new ILPMP dashboard provides a valuable summary of information such as the total prescriptions, total prescribers, and total pharmacies for that patient within the last year. You can also quickly identify if that patient currently has prescriptions with a cumulative morphine equivalent daily dose (MEDD) of over 90 mg, currently has overlapping opioid prescriptions or overlapping opioid and benzodiazepine prescriptions, or if a patient has received a long-acting opioid while previously considered opioid-naïve (See Figure 1). A patient may be considered opioid-naïve if they have been on less than 60 mg of oral morphine, or its equivalent, for a duration of seven days or less. One newer feature that is perhaps the most useful is the “mapping prescriptions” function, which allows for geo-mapping of patient address, prescriber address, and pharmacy address (See Figure 2). Keep in mind that all this data represents a point on the map and doesn’t necessarily confirm substance abuse or diversion. Occasionally information in the ILPMP can be incorrect, therefore it is always recommended that prescribers and dispensers confirm the available ILPMP information with the patient.
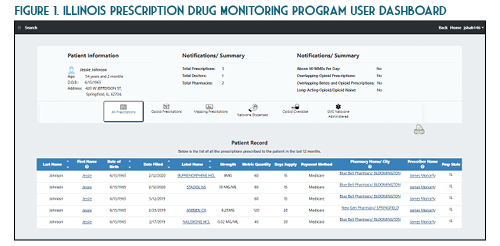
In the State of Illinois, a prescriber (or their designee) must document an attempt to access the ILPMP prior to providing a prescription for an initial Schedule II narcotic prescription (720 ILCS 570/314.5). At this time pharmacists are not required to document an attempt to access the ILPMP. However, based on the pharmacist’s “corresponding liability” under the Federal Controlled Substances Act, this practice is highly encouraged. While the law requires accessing the ILPMP only for the initial prescription, in practice, this should be performed (and documented) prior to each prescription from a patient safety and medico-legal standpoint. The requirement to document an attempt to access the ILPMP prior to issuing an initial Schedule II opioid prescription does not apply to the inpatient setting, patients receiving active oncology treatment, palliative care / hospice patients, or patients receiving a seven day or less supply from an emergency department.
On January 1st, 2018, Illinois Public Act 100-0564 mandated that all electronic health record (EHR) systems utilized within Illinois interface directly with the ILPMP on or before January 1st, 2021. The integration of the PMP into the electronic health record will enable the prescriber to view the PMP data without leaving their workflow and logging into the ILPMP website. Utilizing this integration, also known as PMPnow, is anticipated to save time and money. The PMPnow integration can be requested through the ILPMP at no cost to the health-system or pharmacy, although the EHR or pharmacy software vendor may charge the system for upfront costs associated with establishing the ILPMP connection. Currently ILPMP is integrated with approximately 250 pharmacies. Prescribers and pharmacists should work with their EHR and pharmacy software vendor to ensure they are meeting stale law requirements. The connection between the ILPMP and your respective EHR is encrypted to ensure patient confidentiality.
Yes. If you are a prescriber or pharmacist directly involved in the care of a patient, then accessing the ILPMP data and communicating that data with another professional directly involved in the care of the patient would fall under the HIPAA definition of “treatment.” For instance, if you are a pharmacist and note that one of your patients is receiving alprazolam from his psychiatrist and his primary care physician, calling both prescribers would be considered reasonable under the HIPAA definition. There is some ambiguity, however, if you do not have an established relationship with the patient. Perhaps a new patient comes to your pharmacy with a prescription for hydrocodone. You refuse to fill the prescription due to concerning information on the ILPMP. Calling the prescribers of this patient may not be covered under the HIPAA definition of “treatment.”
You are a community pharmacist in a large retail chain. A patient well known to you approaches the counter with a prescription for fentanyl transdermal patch 75 mcg/hour. You review his prescription profile and note a routine monthly prescription for oxycodone / acetaminophen oral tablets 5-325 mg with instructions to take 1 tablet PO every 8 hours as needed for severe pain. While you know a prior authorization will be required for the patient’s prescription insurance, you ask your pharmacy student what they would like to do.
The student recommends first asking the patient if they have been on the fentanyl patch prior and if so, what strength and how long ago. The patient denies prior experience with fentanyl patches. You ask your stellar student if this patient is opioid-naïve or opioid tolerant. The student notes that the patient routinely fills the oxycodone tablets each month, assuming that they use the three allowed doses each day. This would be 15 mg of oral oxycodone which is equivalent to approximately 20 mg of oral morphine equivalents daily (MEDD). While the patient has been on this therapy for several years, the MEDD is less than 60 mg, which means this patient should be classified as opioid-naïve. The safety of the fentanyl patch for this patient should be questioned. You and your student log on to the ILPMP and note that this patient has been receiving oral controlled release morphine 60 mg dosed every 12 hours from the same prescriber, but it is filled at a different pharmacy. This would place the patient in the opioid-tolerant category, but the different pharmacy certainly raises a red flag. You mention this when you call the prescriber. Before the patient leaves, the student asks, “what about naloxone?” and suggests to the pharmacist that if the patient does not have a dose of naloxone available at home, they should consider obtaining one per the state-wide standing order while providing the standardized procedures for administration.
You are a hospital pharmacist working in the emergency department. Your hospital has recently integrated PMPnow into your electronic health record. A patient presents for uncontrolled low back pain due to a fall and is requesting something for severe pain. You review this patient’s ILPMP record and note that they are routinely using fentanyl transdermal patches. A urine drug screen is positive for an “opiate.” The emergency physician comes by on her way to see the patient and stops to ask you your thoughts.
First and foremost, the ILPMP should be used to improve patient care, not as punitive action. You are to be commended for reviewing the ILPMP. However, because this patient falls under one of the exempt categories, documenting the attempt to access the ILPMP is not legally-mandated should you choose to send this patient out with a prescription for an opioid analgesic. The other concern is the positive drug screen for “opiate.” Traditionally immunoassay urine drug screens are not sensitive for synthetic or semisynthetic opioids unless specifically stated. The first conclusion here would be the patient is using an illicit opioid. That certainly is a distinct possibility, but an additional consideration could be that this patient received an opioid analgesic in another emergency department or hospital recently. This would not be reported to the ILPMP.
The Illinois Prescription Drug Monitoring Program is a valuable tool for prescribers, pharmacists, and pharmacy technicians. Routine review of the ILPMP is essential for improved clinical decision making, safer opioid prescribing, and improved patient outcomes while reducing opioid misuse, abuse and overdose. Both prescribers and pharmacists may designate up to three licensed designees to query the database on their behalf. Pharmacists should be prepared to discuss ILPMP findings or concerns with both patients and prescribers. Pharmacy technicians should be prepared to discuss ILPMP findings with a pharmacist.
Sarah Pointer, PharmD
401 North Fourth Street
Springfield, IL 62702
https://www.ilpmp.org/index.php
https://www.ilpmp.org/PMPnowRegistration.php
- Gugelmann HM, Perrone J. Can prescription drug monitoring programs help limit opioid abuse? J Am Med Assoc. 2011;306(20):2258-2259. doi:10.1001/jama.2011.1712.
- Reifler LM, Droz D, Bailey JE, et al. Do prescription monitoring programs impact state trends in opioid abuse/misuse? Pain Med. 2012;13(3):434-442. doi:10.1111/j.1526-4637.2012.01327.x.
- Fink DS, Schleimer JP, Sarvet A, et al. Association between prescription drug monitoring programs and Nonfatal and Fatal Drug Overdoses: A Systematic Review. Ann Intern Med. 2018;168(11):783-790. doi:10.7326/M17-3074.
- Centers for Disease Control and Prevention. 2019 Annual surveillance report of drug-related risks and outcomes — United States surveillance special report. Centers for Disease Control and Prevention, U.S. Department of Health and Human Services. Published November 1, 2019. Accessed 2020 Apr 2020 from https://www. cdc.gov/drugoverdose/pdf/ pubs/2019-cdc-drug-surveillance? report.pdf.

College Connection
Midwestern University Chicago College of Pharmacy
How Working in a Hospital Pharmacy Changed my Perspective as a Student
College Connection
by Breanna Failla, PS-2, ICHP Member, Midwestern University Chicago College of Pharmacy
Roosevelt University College of Pharmacy
Preparing the First Year Pharmacy Students for Success: Integrated Sequence (IS) Workshop and Making Biostatistics Fun
College Connection
by Jeremy Fernandez Balingit, PS-3, SSHP President Roosevelt University College of Pharmacy
Rosalind Franklin University of Medicine and Science College of Pharmacy
ICHP Fundraising Success Throughout the Academic Year
College Connection
by Marie Aquilino, PharmD Candidate 2022, ICHP Fundraising Chair
Another successful event was a t-shirt fundraiser. With the help of our creative executive board, we came up with a great design and made sure to market the shirt to each class in the college. Once designed, the shirts nearly sold themselves. The driving factor was the individuality and quality of our t-shirts. Execution and advertising were vital to student body engagement and the success of the fundraiser.
With new fundraising ideas, substantial planning must occur. With no prior guidance or protocols, the planning process can be daunting and time consuming. Advertising should begin well before the event takes place and should be consistent until the event occurs. Advertising on campus can range from distributing flyers, sending college or university wide emails, utilizing social media, strategically placing the event in a high traffic area, and planning an appropriate date and time.
Southern Illinois University Edwardsville (SIUE) - School of Pharmacy
Pharmacy Across the Ocean
College Connection
by Justin Shiau, P2, President-Elect, Southern Illinois University Edwardsville (SIUE) - School of Pharmacy (SOP) and Catherine Gilmore, PharmD Candidate 2020, Southern Illinois University Edwardsville (SIUE) - School of Pharmacy (SOP)

A: Traveling to India and experiencing pharmacy practice there was such a wonderful opportunity! We were able to spend 2 weeks at a private, 1800-bed hospital and see how they treated a variety of disease states, such as Dengue Fever, thalassemia, multi-drug resistant tuberculosis, and pediatric endocarditis. Since it was a teaching hospital, we were able to round with attendings, medical residents, medical students, and pharmacy students. We didn’t prepare for rounds each morning because there was only one paper chart available on each patient, so recommendations for changes in therapy came after rounds when chart was available. We then split the next week at a government-run cancer hospital and a government-run HIV hospital. We finished our time at a rural, government-run hospital at a hill station in the mountains, where most people were being treated for COPD. Here, the government supplies all medications free of charge. Pharmacists are involved when it comes to recommending medications on the government’s formulary and obtaining non-formulary medications when indicated. It was such a different experience than a medicine/hospital APPE rotation in America because while the treatment options are more limited the cost is so low.
A: The PharmD degree is very new in India. The first cohort of PharmD students graduated in 2014, so clinical pharmacy is growing. Before, students received a Bachelors or Masters in pharmacy (and they still can), with most graduates pursuing a career in industry. They also have a Diploma in Pharmacy, which is an entry-level degree that takes approximately 2 years to earn, and is for those who want to practice more community-based pharmacy. From my understanding, there is not much clinical information taught with this degree and it is mostly how to run your own pharmacy. This degree was necessary for citizens in rural areas to have better access to medications. As the PharmD degree grows and more graduates are seeking advanced degrees, it is the intent of the Pharmacy Council of India to phase out the diploma program.
A: Prescription drug prices. Hands down. Medications are so incredibly cheap! We were in one of the pharmacies at the hospital looking at the various inhalers they had, and I picked up a budesonide/formoterol inhaler. I asked the pharmacy clerk how much the inhaler cost and she said “600 rupees.” I whipped out my calculator and converted that to dollars—it was a little over $8. And that’s the price the patient pays. Having insurance is not as common in India as it is in America, but the cost of healthcare is very low and fairly affordable for all patients. It is crazy that this same branded inhaler is over $300 in America, but is a fraction of the price in India.
A: I would definitely recommend any global opportunity. It helps you to understand what pharmacy practice is like in other countries and how culture and religious beliefs can shape healthcare globally. This experience was unique for me since almost everyone I came into contact with spoke English, so language was not as large of a barrier as it might be with other experiences abroad. I think if students can step out of their comfort zone, even for a month or so, it helps you appreciate the things that we take for granted (e.g., toilet paper, drinking tap water, constantly smelling like bug repellant, etc.). Specifically, India also has SO much to offer! Mysore, the city where we spent most of our time, was home to many yoga studios and schools, as they teach Ashtanga Vinyasa yoga there. The south Indian food was absolutely fabulous, and we never had a bad meal. Mysore is home to the Mysore Palace, a beautiful palace open to the public that happens to be the 2nd most visited place in India. We took full advantage of visiting. There is so much to see and do in India and we were never bored!
University of Illinois at Chicago College of Pharmacy
Shifting Landscape in Health-System Pharmacy: USP 797 Impacts of Implementation
College Connection
by Tony Rosella, ICHP Professional Practice Chair, Second-year Student Pharmacist at University of Illinois at Chicago College of Pharmacy
- Compounding Standards, 2020. https://www.usp.org/compounding (accessed 8 March 2020)
- What is USP 797 and How to Stay Compliant, 2019. https://www.pharmacyonesource.com/what-is-usp-797-and-how-to-stay-compliant/ (accessed 8 March 2020)
More
Upcoming Events
May 21, 2020, 12:00 pm
Topic: Implementation of Best Practices for Asthma
Speaker: Lori A. Wilken, PharmD, BCACP, NCTTP, AE-C
Accredited for health-system pharmacists and pharmacy technicians
October 1-3, 2020
Drury Lane Theatre & Conference Center
Oakbrook Terrace, IL
We've got some great home study webinars planned for the coming weeks!
- Educational Affairs
3rd Tuesday of each month - 11:00 am - Executive Committee
1st Tuesday of each month - 8:00 am - Government Affairs
3rd Monday of each month - 5:00 pm - Marketing Affairs
3rd Tuesday of each month - 8:00 am - Organizational Affairs
2nd Wednesday of each month - 3:00 pm - Professional Affairs
4th Tuesday of each month - 3:00 pm - Technology Committee
2nd Friday of each month - 8:00 am
- Ambulatory Care Network
1st Thursday each month - 12:00 pm - Chicago Area Pharmacy Directors Network Dinner Meeting
See ICHP calendar for details: www.ichpnet.org/events/calendar/ - New Practitioners Network
3rd Wednesday of each month - 4:00 pm - Pharmacy Technician Network
2nd Tuesday each month - 5:00 pm - Small and Rural Hospitals Network
See ICHP calendar for details: www.ichpnet.org/events/calendar/
Welcome New Members!
- Sarina Acevedo
- Asma Adamji - Recruited by Jessy Johnson
- Amartsetseg Ajuu - Recruited by Michelle Smith
- Yasser Ali
- Benjamin Allen - Recruited by Erik Stojanoff
- Lara Allison
- Christiana Amegatcher
- Erica Asturizaga
- Danielle Baum
- Lucas Beckman
- Natalie Boelens - Recruited by Elizabeth Harthan
- Kevin Castro - Recruited by Erik Stojanoff
- Chirag Contractor - Recruited by Erik Stojanoff
- Alexander Cuenca
- Lejla Cukovic
- Sarah Faust
- Evan Fetten - Recruited by Erik Stojanoff
- Kari Frommelt
- Damaris Fuentes
- Melissa Gilbertsen
- Eric Gray
- Cory Hall - Recruited Michelle Smith
- Irma Hart
- Ron Hartman - Recruited by Elizabeth Harthan
- Anna Hobel
- Tereka Hunter
- Stephanie Hunziker - Recruited by Carrie Vogler
- Jerry Jacob
- Wiwik Kertayuda
- Hyun Kim - Recruited by Erik Stojanoff
- Jayson Kimura
- Sarah Kinzer - Recruited by Penny Roch
- Jhoanna Lee
- Elizabeth Leo
- Kavina Luna
- Heather Margetich - Recruited by Penny Roch
- Ifleda Millon
- Nadine Ness
- Ashlee Patton
- Joseph Saclolo
- Jaswinder Sandhu
- Alex Sandoval
- Aisha Shajee
- Melissa Shively - Recruited by Oksana Kucher
- Skytoria Smith
- Jessica Smith
- Alleshea Snell
- Alexis Soto Cardenas
- Christine Stankus
- Katarzyna Szaflarska
- Lashonda Tate
- Ryan Truong - Recruited by Erik Stojanoff
- Ashley Valencia
- Donna Vazirnia - Recruited by Erik Stojanoff
- Amela Vrselj
- Lisa Wall
- Joshua Wentland
- Mark Wilson
- Anthony Xavier
- Dagmara Zajac
- Valerie Zona-Baxter
- Abigail Auer
- Michele Averhart
- Nickita Benny
- Nancy Castro
- Kaleia Collins
- Christopher Fontillas - Recruited by Milena Murray
- Jacquelyn Grimm
- Bethany Harris - Recruited by Erin Hatfield
- Lorraine Johnson
- Alexis Leach
- Kassandra Mason
- Edib Mravic - Recruited by Rebecca Ohrmund
- Adam Procento
- Stephen Salisbury - Recruited by Donald Schaeffer
- Syruliah Shah
- Natasha Shea - Recruited by Kristi Stice
- Victora Simmons - Recruited by Julia Schimmelpfennig
- Margo Stone
- Brian Sutton
- Brittany Yang
- Andrew Zouzani
- Haneen Alomar
- Dan Chen
- Kambi Colvin - Recruited by Michelle Smith
- DeJada Daily - Recruited by Becky Ohrmund
- Margaret Dangerfield
- Jena Deschler - Recruited by Rajul Gandhi
- Amanda Herald - Recruited by Linda Fred
- Sue Jackson - Recruited by Megan Malone
- Saira Jatoi
- Kalombo Kalonji - Recruited by Linda Fred
- Dylan Kist
- Laila Kuziez
- Tilo Lamken - Recruited by Linda Fred
- Eva Morrison - Recruited by David Huhtelin
- Patricia Nasser
- Kirsten Ohler - Recruited by Adam Bursua
- Jacinda Ounkham Recruited by Linda Fred
- Matthew Plassmeier
- Emen Salam - Recruited by Megan Malone
- Kruti Shah - Recruited by Nora Flint
- Tammy Shelly - Recruited by Linda Fred
- Alexa Smalley
- Tanya Sotelo
- Brittani Stone
- Adrian Valadez
ICHP Pharmacy Action Fund (PAC)
.jpg)
Board of Directors, Student Society Presidents & Affiliates
 Carrie Vogler
President
|
 Julie Downen
Regional Director
Central
|
 David Martin
Educational Affairs
Director
|
 Tara Vickery Gorden
Small and Rural Hospital
Network Chair
|
 Noelle Chapman
Immediate Past
President
|
 Alifiya Hyderi
Regional Director
Northern
|
 Bernice Man
Marketing Affairs
Director
|
 David Tjhio
Committee on
Technology Chair
|
 Jen Arnoldi
President-Elect
|
 Jared Sheley
Regional Director
Southern
|
 Sharon Karina
Government Affairs
Director
|
 Jennier Phillips
Editor & Chair,
KeePosted
|
 Christopher Crank
Treasurer
|
 Kristine VanKuiken
Technician
Representative
|
 Natalie Tucker
New Practitioners
Network Chair |
 Milena Murray
Assistant Editor,
KeePosted
|
 Ed Rainville
Secretary
|
 Elise Wozniak
Organizational Affairs
Director
|
 Dan Majerczyk
Ambulatory Care
Network Chair
|
 Nora Flint
Residency Leaders
Network Chair |
 Scott Meyers
Executive Vice
President
ICHP Office |
 Amy Boblitt
Professional Affairs
Director
|
 |
| Sanad Abduljawad Chicago State University College of Pharmacy |
Kristen Ingold Southern Illinois University Edwardsville
School of Pharmacy
|
| Irum Khan Midwestern University Chicago College of Pharmacy |
Josiah Baker University of Illinois at Chicago College of Pharmacy |
Jeremy Fernandez Balingit
Roosevelt University College of Pharmacy
|
Bill Clafshenkel University of Illinois at Chicago Rockford Campus College of Pharmacy
|
| Nimita Shah Rosalind Franklin University College of Pharmacy |
| Milena McLaughlin President |
Andrew Merker President-Elect |
| Denise Kolanczyk Immediate Past President |
Erin Shaughnessy Treasurer |
| David Martin Secretary |
Richard Puccetti Technician Representative |
| Liz Harthan President |
Ed Rainville Immediate Past-President |
| Jared Sheley President |
| Megan Stoller President |
Ashlie Kallal President-Elect |
Billee Samples Immediate Past-President |
President, Southern IL Society
President, Sugar Creek Society