Official Newsjournal of the Illinois Council of Health-System Pharmacists

November 2021
Volume 47 Issue 4
Columns
Educational Affairs Poster Abstracts
Professional Affairs - Call for Entries
Features
College Connection
Chicago State University - College of Pharmacy
Southern Illinois University Edwardsville (SIUE) - School of Pharmacy
University of Illinois at Chicago-Rockford
More
ICHP Pharmacy Action Fund (PAC)
Officers and Board of Directors
 Directly Speaking
Directly Speaking
What We Learned From You!
by Scott A. Meyers, Executive Vice President
In February, all ICHP members should have received a membership needs assessment via e-mail to determine your understanding of ICHP’s advocacy efforts. Just over 11 percent of you responded. Not the best results for any survey but not pathetic either.
At the beginning of the survey, we provided Merriam-Webster’s definition of advocacy: “the act or process of supporting a cause or proposal; the act or process of advocating”, in order to provide clarity on the purpose of the survey. Before we asked the questions, we went on to explain that ICHP’s advocacy efforts included supporting pharmacy related issues in Springfield with the General Assembly and with the Board of Pharmacy and State agencies like the Illinois Department of Financial and Professional Regulation. Advocacy is a big part of what ICHP does but its value and importance seem to be poorly understood by our members.
Let me share some of the results of the survey here in the hope of convincing more of you to increase your personal advocacy efforts and perhaps increase your overall awareness of the advocacy efforts of ICHP. The entire anonymous results of the survey are available on the ICHP website at https://bit.ly/2HK5eeE .
In the survey’s first question, we asked members to pick a statement that best aligns with their interest/perception of ICHP’s advocacy. The results were mostly encouraging.
- 10% of respondents indicated they were very interested in advocacy and involved in ICHP’s efforts.
- Nearly 25% were very interested and wanted to get more involved.
- Unfortunately, 48% were only somewhat interested and felt they had no time to get involved.
- Just over 7% were honest and felt they lacked an understanding of advocacy but were willing to learn more.
- Nearly 8% believed ICHP would advocate for them and there was no need to participate.
- And nearly 3% of respondents had no interest in advocacy.
To the 48%, 8% and 3% who either didn’t have time, didn’t feel the need or didn’t care about advocacy, (and the numbers are probably higher among those who didn’t complete the survey) I would like to urge you to take just a few hours a year to get more involved. Meeting with your legislators once a year is often all it takes to build a useful connection that can help you practice pharmacy better. Maybe not after the first or second meeting, but with no term limits in Illinois after a few years, the legislators will remember you and listen to you because they know you care. And reading the Government Affairs reports in the KeePosted won’t take but a few minutes and Jim Owen and I always highlight the most important bills under consideration. Finally, with ICHP’s Advocacy Center on our website, connecting with your legislator via e-mail has never been easier!
In another section of the survey, we asked members if they had attended a Legislative Day in the past or might in the future. The results were again somewhat surprising and disappointing:
- About 30% have attended a Legislative Day in the past and hope to attend again in the future. (This is encouraging!)
- 9% have attended in the past but will not attend again. (Not so encouraging, we make our impressions by repeated visits either in the Capitol or at home.)
- 32% have not yet participated but hope to in the future. (More encouraging signs from our members!)
- Nearly 30% have not and do not intend to attend a Legislative Day in Springfield! (This is the saddest result.)
When asked why those members indicated that they will not attend in the future, there were a few common reasons. Many were concerned with giving up a vacation day for this important effort, while a few others complained that the $25-50 registration fee for pharmacists was too much money. While we didn’t ask for endorsements of Legislative Day on this survey, past Legislative Day attendee evaluations have praised the experience as enlightening, a great opportunity to see our government in action, a day well-spent, and an opportunity to see that legislators are just like us and need our input to do their jobs. I would encourage the most ardent cynic to give a future Legislative Day a try and if they still believe that it is a waste of time, I will refund their registration fee. Before I came to work for ICHP, I spent countless vacation days and traded many shifts in order to participate in professional events including Legislative Day that I thought were important. I never regretted a single one!
Finally, in the survey, we described the ICHP Pharmacy Action Fund, ICHP’s political action committee (PAC) and asked members if they contributed, planned to contribute, or why they didn’t plan to contribute. Again, some of the answers were disappointing but not necessarily surprising:
- Nearly 11% said they contributed regularly. (Good but that really only amounted to 16 individuals).
- Almost 20% said they had contributed at least once and intended to contribute again in the future. (That’s very encouraging.)
- 28% had not contributed but agree that it is important and will contribute in the future. (Also very encouraging.)
- Not quite 9% felt PACs are unethical and will not contribute. (A little discouraging but political contributions are a way of life in Illinois and the U.S.)
- What is perhaps the saddest result is that 30% of respondents were unaware that ICHP has a PAC, but the good news is that they will consider contributing in the future.
- Separately, around 25% felt that ICHP should publish the names of the legislators who receive Pharmacy Action Fund moneys be published in the KeePosted. (A great suggestion which will probably be implemented.)
- 10% of the respondents felt the names of member contributors should be published in the KeePosted, and in fact, they are!
The ICHP Pharmacy Action Fund is a valuable advocacy tool and while we don’t raise or contribute the large contributions of many PACs in Illinois, the contributions we make have made a difference and have demonstrated to the legislative leaders that we are serious in our efforts. These contributions open doors so our issues can be heard and once heard, many of those issues get traction and acceptance with General Assembly leaders. Just like life in general, without some skin in the game, your gains will be very, very limited.
I encourage each of you to take a few minutes and read the results of our advocacy survey and then spend a few more minutes considering what advocacy efforts you can personally undertake for your profession. I think if you seriously believe you belong to a worthwhile profession, you will find a few ways that you can up your advocacy game without spending a lot of time, money and effort. And you’ll be surprised how easy it is!
 President's Message
President's Message
Elevate your Team
by Travis Hunerdosse, PharmD, MBA, ICHP President
Healthcare is a complex system made up of multiple teams that enable the delivery of care and services to patients. All of us work as a member of many teams - patient care teams made up of healthcare professionals in hospitals and ambulatory clinics; pharmacy teams that work synchronously to provide expert medication management and drug delivery services; and quality and safety teams to ensure care is delivered safely and effectively to all patients throughout the health system.
A team is a group of individuals who possess complementary skills that are required to complete a task, job, or project. Members of a team work independently, share responsibility, are accountable for outcomes and performance, and work toward a common goal. Effective teams are those that are more than just a collection of people performing tasks to merely get the job done. Teams that have a strong sense of culture and mutual commitment create a synergy that allows the team to generate high performance that is much greater than the performance of individuals. All team members, technicians, pharmacists, big L, and little l leaders influence the performance of the team and can elevate individual members of that team to achieve greatness.
Being in a formal leadership position is not a requirement to have an impact on a team and its individual members. Little l leadership is required from the team members in order for the team to perform at its best. For example, senior technicians are responsible for training and mentoring of newly hired teammates. This is a vitally important role to ensure medications are prepared and made available in a safe and efficient manner. Similarly, clinical pharmacists serve as primary preceptors for pharmacy students, residents, and new practitioners. In the preceptor role, the clinical pharmacist is responsible for shaping the future of the profession and serving as a role model. A strong team requires individuals to step up and assume responsibility for elevating the people surrounding them.
At this point, you may be asking yourself “how can I elevate members of my team”? Here are some tips and fundamental to elevating the people around you.
Give them face time.
In order to elevate your team, it is important to let them take center stage. In your role, think of how you can increase your teammate’s interactions with other healthcare providers and leaders in the organization. For example, pharmacy students should present their recommendations to the physician and care team during rounds based on an earlier discussion with the preceptor. If you have been asked to give a presentation at a department or committee meeting, provide the opportunity for team members to get involved in delivering the presentation.
Remind them that they are the experts.
Some team members may be a little hesitant to take center stage. They may need some encouragement and a reminder that they have the skills and the training to do a great job. It is important for team members to have confidence in their role and to establish strong relationships with key customers and stakeholders.
Pave the way.
It is important that your customers and stakeholders understand why they may be interacting with your team rather than you directly; or why you are providing the opportunity for your team member to present at a meeting. This also demonstrates to your team and customers that you trust the talents of your team members.
Practice. Practice. Practice.
You need to ensure your team is prepared to deliver recommendations or presentations. This means you have to take the time to practice with your team on how to have those interactions with your customers. In order to have a positive outcome, you need to provide clear direction to the team and coach them on delivery of the message. This will also allow you to provide feedback and instill confidence in the team member.
Elevating your team also means finding the right people, setting the culture, and leading by example. These fundamentals will ensure your team is performing at the top of their ability.
- Pick the right people – make sure you have the right person in the right seat. Outline the key responsibilities of the role and hold the team accountable.
- Inspire – inspire the people around you to work at the top of their ability. Provide the inspiration to take their practice to the next level.
- Celebrate – take the time to celebrate the wins your team achieves. Showing appreciation for a job well done keeps everyone motivated to continue pushing forward.
- What is their “why”? – take the time to remind the team of their “whys”. Why did they decide to be a pharmacy technician or clinical pharmacist? Why do they have a passion for it? What are they hoping to achieve?
Preparing the team to practice at the top of their ability and advance to new roles is every team member’s responsibility. Fostering teamwork, collaboration, and excellence is key for a high performing team. I encourage you to take the time to reflect on how you can use these tips to impact your team and elevate their performance.
References:
Jaycox, C. Elevate your value by elevating your team. Forbes. March 5, 2018. https://www.forbes.com/sites/forbesbusinessdevelopmentcouncil/2018/03/05/elevate-your-value-by-elevating-your-team/2/#eec9a30a6074 Accessed April 22, 2018.
Pentecost, M. 4 ways to elevate your team. The Business Journals. December 5, 2017. https://www.bizjournals.com/bizjournals/how-to/human-resources/2017/12/4-ways-to-elevate-your-team.html Accessed April 22, 2018.
Columns
Leadership Profile
ICHP Spotlight Interview: Christopher W Crank

Leadership position in ICHP
Special thanks for making me who I am today in my career
Advice for a student
Applying ICHP learning into my work
Challenges that I face at my practice
My special interests or hobbies outside of work
My favorite project so far was making a memory chest for a friend of mine. Her grandson passed away in the NICU, and she wanted to give her daughter a chest to keep all of her keepsakes from the baby. I was honored and humbled that she asked, but I was also extremely nervous about how it would turn out. The chest turned out well, and her daughter loved it. It will likely be impossible for me to make something with more meaning.
3 adjectives people use to describe me
Educational Affairs
Alternatives in the Management of Cardiac Toxicity from Tricyclic Antidepressant Overdose in Light of the Present Intravenous Sodium Bicarbonate Shortage
by Jaxson Burkins, PharmD; PGY2 Emergency Medicine Pharmacy Resident, Rush University Medical Center; Anthony M Burda, BSPharm, DABAT; Clinical Toxicologist, Illinois Poison Center; Carol DesLauriers, PharmD, DABAT; Senior Director, Illinois Poison Center
This drug shortage presents a challenge for the medical community as alternate therapeutic options are needed when intravenous sodium bicarbonate is unavailable. At toxic doses, TCAs cause a blockade of the fast voltage-gated sodium channel in the myocardium, which results in a widening of the QRS complex on an electrocardiogram (EKG). Other EKG abnormalities include rightward shift of the QRS axis, rightward shift of the terminal 40 msec of the QRS complex, and an R-wave of 3 mm or greater in aVR. Ultimately, these EKG changes and cardiac disturbances can result in dysrhythmias. Intravenous sodium bicarbonate provides two mechanisms to correct this widened QRS complex. First, sodium bicarbonate provides additional sodium ions to overcome the channel blockade, which in turn shortens the QRS interval, provides cardiac stability, and may increase blood pressure. Sodium bicarbonate also potentially offers serum alkalization via chemical buffering and altering the binding of the toxin to the receptor site. Additionally, the increased pH enhances the protein binding of TCAs, decreasing the amount of free drug available to bind to receptors. In severe overdoses, the general dosing is 1-2 mEq/kg to target an arterial pH of 7.45-7.55 and a QRS duration of < 120 msec. While sodium bicarbonate is a first-line therapy in TCA overdoses, given the current drug shortage, it is important to be aware of possible alternatives, which are summarized below.
Hypertonic Saline
Hyperventilation (if intubated)
Intravenous Lipid Emulsion
Sodium Acetate
Lidocaine
During drug shortages, pharmacists are often asked for therapeutic alternatives to medications in short supply. While intravenous sodium bicarbonate plays an important role in the treatment of overdoses, there are therapeutic alternatives that may be considered when the drug is unavailable. For more information, please contact the Illinois Poison Center at (800) 222-1222.
References
Educational Affairs Poster Abstracts
2018 ICHP Spring Meeting Poster Abstracts
EDITOR’S NOTE: All handouts for presentations at the 2018 ICHP Spring Meeting may be found on the Spring Meeting handouts page. https://www.ichpnet.org/events/spring_meeting/2018/handouts/2018_spring_meeting_handouts.php
Please note: All research was to have results and conclusions by the time of presentation. This may not be reflected in the posted abstracts below.
PLATFORM PRESENTATIONS
1. Assessment of Automated Usage Forms for Controlled Substance Tracking at a Large Academic Medical Center
2. Evaluation of intraoperative, local site injections of liposomal bupivacaine as an alternative to standard local anesthetics in patients undergoing total hip arthroplasty
ORIGINAL
1. Impact of Using "Load on Demand" with Automated Dispensing Machines (Presented as Poster #1)
2. Evaluating the effects of dexmedetomidine in addition to a standard benzodiazepine protocol in patients with alcohol withdrawal admitted to the intensive care unit (Presented as Poster #2)
3. Evaluation of acute kidney injury with mannitol versus hypertonic saline in the treatment of elevated intracranial pressure (Presented as Poster #3)
4. Impact of Ketamine in the Management of Painful Sickle Cell Disease Vaso-Occlusive Crisis (Presented as Poster #4)
5. Time Within Therapeutic Range: A Comparison of Three Tacrolimus Formulations in Renal Transplant Recipients (Presented as Poster #5)
6. Impact of psychological debriefing on the mental health of pharmacy residents participating in a 24-hour, in-house clinical pharmacy on-call program (Presented as Poster #6)
7. Impact of a PharmD Consult to Improve Futile Medication Discontinuation Rates in a Home Hospice Program (Presented as Poster #7)
8. Retrospective institutional assessment of vancomycin dosing protocol on the attainment of goal serum trough concentrations in the pediatric setting (Presented as Poster #8)
9. Evaluation of Outpatient Infusion Medication Safety Monitoring at a Community Teaching Hospital (Presented as Poster #12)
10. Implementation of a Pharmacist-Directed Medication Therapy Management Service for Oral Chemotherapy Patients at an Outpatient Cancer Center (Presented as Poster #13)
ENCORE
1. Use of a dashboard for monitoring
clinical pharmacy services in a large medical center (Presented as Poster #9)
2. Improving compliance with inpatient
utilization of medications that have Elements to Assure Safe Use at a large
academic medical center (Presented as Poster #10)
3. Implementation of phone etiquette
and triage training in pharmacy technicians at a large academic medical center
(Presented as Poster #11)
4. Active Pharmacy Student Engagement
in Inter-Professional Education (Presented as Poster #14)
5. Identifying Barriers to Performance
of a Newly Implemented Spontaneous Awakening Trial Protocol (Presented as Poster
#15)
6. Evaluation of standardized drug
concentrations for intravenous adult continuous infusions (Presented as Poster #16)
7. Implementation and evaluation of an
error and complaint reporting system in a specialty and ambulatory care
pharmacy (Presented as Poster #17)
8. Feasibility and impact of
bortezomib batching in a high-volume outpatient oncology clinic ({Presented as Poster
#18)
STUDENT
1. Amoxicillin dosing frequency in pediatric patients diagnosed with community acquired pneumonia and its effect on hospital readmission rates. (Was to be presented as Poster #19 – not presented)
2. Pneumococcal Vaccination in Pediatric Cystic Fibrosis Patients (Presented as Poster #20)
**********************************************************************
1. ICHP Poster Presentations - Platform Presentation
Category: Encore
Title: Assessment of Automated Usage Forms for Controlled Substance Tracking at a Large Academic Medical Center
Abstract:
Purpose: Maintaining tight regulation of
controlled substances within an institution is imperative for the safety of
patients and employees as well as for the prevention of diversion. At a large academic medical center, a
paper-based tracking system is currently used for controlled substances that
are dispensed from the pharmacy for patient-specific doses. This manual process is outdated and requires
archiving the paper log sheets used for tracking. An automated system is in place at this
medical center that has an alternative way for tracking patient-specific
controlled substances that are dispensed from the pharmacy via usage forms. The goal of this project is to pilot
implementation of controlled substance usage forms for controlled substances
that are dispensed from the pharmacy for patient-specific doses.
Methods: Two units within a large academic medical center will pilot the controlled substance usage forms for two weeks. During this pilot, the existing paper-based tracking system will be continued in conjunction with the new usage form process. The automated system generates a usage form which is specific to the patient and the controlled substance being dispensed. These usage forms will allow nurses to document when a medication is administered, wasted, or returned. This form will then be returned to the pharmacy and reconciled back into the automated system resulting in an electronic record. The following information will be collected to assess the feasibility of this new process: complete usage forms, incomplete usage forms, missing usage forms, and pharmacy technician time spent reconciling forms. After the pilot is complete a simple survey will be given to nurses to evaluate overall nursing satisfaction with the usage forms.
Results: Approximately 1 of 4 usage forms was not returned to pharmacy. Almost 50% of the total dispensed usage forms were either missing or incomplete, n=35 (48%). This pilot required a great deal of education for both nursing and pharmacy staff. Pharmacy technicians did not require much time to reconcile automated usage forms into the automated controlled substance system. Pharmacy technicians did not reconcile the usage forms to the electronic medical record in the time estimates. Decentralized pharmacy technicians dedicated to a specific area were crucial in the success of this process. A retrospective audit of the administration of controlled substances in the electronic medical record was conducted by a pharmacist as a part of this study. This is currently isn’t a part of daily practice.
Conclusions: The implementation of hospital-wide automated usage forms at a large academic medical center, especially in units without a dedicated technician, would likely result in similar low rates of compliance. Current pharmacy technician resources would be a barrier to implementation hospital-wide. The survey of nurses post pilot study was not positive overall.
Submitting Author: Daniel Dickson
Organization: Northwestern Memorial Hospital
Authors: Christie Bertram PharmD Erin St. Angelo PharmD Elise Wozniak PharmD all Northwestern Memorial Hospital
************************************************************************
2. ICHP Poster Presentations - Platform Presentation
Winner: Best Platform Presentation 2018
Category: Encore
Title: Evaluation of intraoperative, local site injections of liposomal bupivacaine as an alternative to standard local anesthetics in patients undergoing total hip arthroplasty
Abstract:
Introduction / Purpose: Liposomal bupivacaine
(LB) has a longer duration of action and thus could be used as an alternative
to standard treatment to better control postoperative pain and improve other
parameters. The purpose of this study
was to evaluate the clinical outcomes of LB versus standard therapy in total
hip arthroplasty (THA).
Methods: This was a retrospective cohort study of patients undergoing THA at 3 hospitals from January 2013 to July 2016. The control group received the standard of care ( plain bupivacaine or ropivacaine), while the LB group received a 20 ml injection as a part of their revised regimen. Length of stay (LOS), discharge status, pain scores, opioid consumption, adverse effects, physical function, costs, and payments were compared. Following univariate analyses, multivariable generalized linear models were used to estimate the effects while controlling for patient factors.
Results: 70 patients received LB and 103 were in the control group. The demographics and opioid consumption were similar between groups. The LB group had a shorter LOS and walked further on the day of surgery and first post-op day. Within the first 3 days of surgery, the control group was more likely to use metoclopramide, while the LB group were prescribed more laxatives. No significant difference between the two groups was found in discharge status and pain scores. The LB group had lower average total hospital cost per case ($16,399 vs. $17,716) compared to the control group. No statistical differences were found between both groups at 30-, 60- and 90-days post discharge related to readmissions or payments.
Conclusions: This study shows that LB as an alternate to plain bupivacaine or ropivacaine significantly reduced LOS and improved physical function in patients undergoing THA. The LB group showed lower in-patient total costs and similar 90-day post-discharge readmissions and payments compared to the control group.
Submitting Author: Daniel Knolhoff
Organization: OSF Saint Anthony Medical Center
Authors: Ed Rainville, MSPharm; Carl Asche, PhD; Jinma Ren, PhD; Minchul Kim, PhD; Meagin McManus, OTR/L, MOT; Daniel Knolhoff, PharmD; Brian T. Maurer, MD; Susan Peterson, MS, RN; Jason Weinberg, MS; Lucas Walker, Kyle Shick, PharmD
*********************************************************************
1. ICHP Poster Presentations – Original
(Presented as Poster #1)
Title: Impact on Missing Medications by Using "Load on Demand" with Automated Dispensing Machines
Abstract:
Purpose: ‘Load on Demand’ (LOD) is the function
of adding new medications to automated dispensing machines (ADM), if not
already stocked, immediately after new orders are verified by a
pharmacist. This study assessed the impact
of LOD on medication security, availability of medications at the point of
care, requests for replacement of missing medications, and the number of
dispensed medications associated with the current system using 24-hour
medication fills.
Method: This study involved a 628 bed medical center hospital with 125 ADMs and complete pharmacy services provided 24 hours a day. Implementation of LOD and data collection was conducted on adult patient units from August 1, 2017 to February 28, 2018 (7 months). Data collection included number of ADM dispenses, number of medications included in 24-hour medication fills, and number of missing medication requests from patient care units.
Results: Missing medication doses per week decreased from a baseline of 354 to a 3-month average of 238, or 32.8%. Twenty-four hour medication fills decreased from 379 to 81 for a 78.6% reduction. ADM dispenses increased from 72% to 90%. Reduction in doses dispensed using the 24-hr medication fills along with increased ADM dispenses resulted in improved medication security. These results were achieved with no change in ADM stock-outs.
Conclusion: This study shows that LOD reduces missing medications and improves medication security with no increase in staff and minimal change in workflow.
Submitting Author: Marilyn Garrett
Organization: OSF Saint Francis Medical Center
Authors: Marilyn E Garrett, CPhT Pharmacy Technician Supervisor OSF Saint Francis Medical Center
*********************************************************************
2. ICHP Poster Presentations – Original
(Presented as Poster #2)
Title: Evaluating the effects of dexmedetomidine in addition to a standard benzodiazepine protocol in patients with alcohol withdrawal admitted to the intensive care unit
Abstract:
Purpose:
Alcohol withdrawal develops upon abrupt discontinuation of alcohol
exposure. Severe withdrawal may include
development of agitation, seizures, and delirium tremens. Patients experiencing alcohol withdrawal
symptoms are frequently monitored using the Clinical Institute Withdrawal
Assessment for Alcohol, revised (CIWA-Ar) scale. This scale is utilized to rank
alcohol withdrawal severity. The World
Health Organization recommends benzodiazepines as the mainstay of alcohol
withdrawal treatment(1). Occasionally,
benzodiazepines do not provide adequate symptom relief and patients need
additional sedation. The purpose of this study is to compare clinical outcomes
between those who receive benzodiazepines alone and those who receive
benzodiazepines and dexmedetomidine for alcohol withdrawal.
Methods: This single-center retrospective chart review has been approved by the local Institutional Review Board. Patients admitted to any intensive care unit (ICU) between July 1, 2015 and July 31, 2017 with a diagnosis of alcohol withdrawal syndrome or delirium tremens and initiated on the CIWA protocol will be included. Data will be collected and reviewed based on two groups: those who received benzodiazepines utilizing the CIWA-Ar protocol compared to those who received benzodiazepines utilizing the CIWA-Ar protocol with the addition of dexmedetomidine. Patients will be excluded based on age less than 18 or greater than 89 years, currently pregnant or breastfeeding, those with known allergies to the study medications, or fewer than five CIWA-Ar scores documented. The primary outcome being evaluated is the time to achieve CIWA-Ar score of less than 16 during ICU stay. Secondary outcomes include maximum change in CIWA-Ar score, time to achievement of CIWA-Ar score less than 9, hospital length of stay, ICU length of stay, average Richmond Agitation-Sedation Scale (RASS) score, incidence of delirium tremens, and total cost. Safety endpoints that will be evaluated are hypotension, bradycardia, respiratory depression, and incidence of intubation.
Results: Research in Progress
Conclusions: Research in Progress
Submitting Author: Sarah Wagner
Organization: Memorial Medical Center
Authors: Sarah Wagner, PharmD, PGY-1 Pharmacy Practice Resident, Memorial Medical Center Megan Metzke, Pharm.D., BCPS, Critical Care Clinical Pharmacist, Memorial Medical Center Billee John, Pharm.D., BCPS, Staff Pharmacist, Memorial Medical Center Don Ferrill, Pharm.D., BCPS, Manager, Pharmacy Education & Clinical Services, & PGY-1 Residency Director, Memorial Medical Center
*********************************************************************
3. ICHP Poster Presentations – Original
(Presented as Poster #3)
Title: Evaluation of acute kidney injury with mannitol versus hypertonic saline in the treatment of elevated intracranial pressure
Abstract:
Purpose: In the neurocritical care population,
management of elevated intracranial pressure is crucial to reduce morbidity and
mortality. A pharmacologic option is hyperosmolar therapy with mannitol or
hypertonic saline, but guidelines do not have a preferred agent. Studies have
shown a risk of acute kidney injury with hypertonic saline, a concern
established for mannitol. History of acute kidney injury can affect prognosis
and progression to chronic kidney disease. No studies have compared the safety
of these agents, and due to their effects on the kidneys, this study aims to
evaluate the incidence of acute kidney injury with hypertonic saline and
mannitol.
Methods: This study will be submitted to the Institutional Review Board for approval. A retrospective chart review will identify patients with traumatic brain injury, intracerebral hemorrhage, subarachnoid hemorrhage, ischemic stroke, malignant MCA syndrome, or cerebral edema who have been treated with mannitol or 3% hypertonic saline for elevated intracranial pressure. Exclusion criteria include patients with end stage renal disease or acute kidney injury upon admission and patients 17 and younger. Any patient who received greater than 24 hours of mannitol prior to hypertonic saline or hypertonic saline prior to mannitol will be excluded. The following baseline data will be collected: age, gender, blood pressure, electrolyte levels, acute physiology and chronic health evaluation (APACHE) score, Glasgow Coma Scale, and duration of hyperosmolar therapy. Past medical history collected will include diabetes, heart failure with reduced ejection fraction, chronic kidney disease, and hypertension. Data about concurrent nephrotoxic agents and vasopressor use will also be obtained. The primary outcome will be the incidence of acute kidney injury as defined by the Kidney Disease Improving Global Outcomes guidelines. Secondary outcomes include stage of acute kidney injury, length of stay, return to baseline renal function, dialysis requirements, and incidence of hypotension. A separate multivariate logistic regression analyses for acute kidney injury will be performed to identify predictors from the following: moderate hyperchloremia, hypernatermia, days with serum sodium >145 mmol/L, metabolic acidosis, low serum bicarbonate, elevated osmolarity, elevated osmolar gap, and percent rise of the following in the first three days of therapy: sodium, chloride, serum osmolarity, and osmlar gap.
Results: Research in Progress
Conclusions: Research in Progress
Submitting Author: Rina Patel
Organization: Memorial Medical Center
Authors: Rina Patel, PharmD, PGY1 Pharmacy Practice Resident, Memorial Medical Center Katelyn Conklen, Pharm.D., BCPS, Critical Care/Trauma Clinical Pharmacist, Memorial Medical Center Don Ferrill, PharmD, BCPS, Manager, Pharmacy Education & Clinical Services & PGY-1 Residency Director, Memorial Medical Center
*********************************************************************
4. ICHP Poster Presentations – Original
(Presented as Poster #4)
Title: Impact of Ketamine in the Management of Painful Sickle Cell Disease Vaso-Occlusive Crisis
Abstract:
Purpose: Painful vaso-occlusive crises (VOC) are
the hallmark symptom associated with adult sickle cell disease (SCD) often
refractory to standard treatment.
Opioids while considered the mainstay of therapy, often offer little
relief, even in the setting of escalating doses. These characteristics make
caring for these patients challenging and a set up for rapid onset opioid
induced hyperalgesia (OIH).
The NMDA-receptor has been implicated as a driver in the development of OIH resulting in downregulation of the mu-opioid receptor, and thus refractoriness to traditional mu-opioid agonists. Ketamine a non-competitive NMDA receptor antagonist has demonstrated analgesic properties, and reported to potentially counteract these neuromodulatory changes observed in OIH. At subanesthetic doses, ketamine can help to reduce central sensitization and recouple the effects of opioids in the spinal cord to reduce opioid exposure and improve analgesic effects. Ketamine has demonstrated positive effects in cancer pain and surgical pain management as an adjuvant to opioids to help control pain, but there is little published data surrounding the use of ketamine in SCD patients. The purpose of this study is to evaluate whether the use of ketamine as part of the treatment for VOC will impact length of stay as well as contribute to the body of literature available regarding efficacy and safety of ketamine in adults with SCD with VOC pain.
Methods: This single-center, retrospective, observational study has been approved by the UCM Institutional Review Board. The UCM electronic medical record was utilized to identify the study population of adult patients 18 years of age and older admitted to the UCM adult hospital that had a diagnosis of vaso-occlusive crisis and received a ketamine infusion to help manage their pain from January 1, 2014 through June 30, 2017. Twenty-four admissions were included in the analysis from 12 unique patients.
Results: The primary endpoint comparing the length of stay when ketamine was employed compared to previous admissions where the same patient did not receive ketamine was numerically lower in the admission where ketamine was used but did not reach statistical significance (13.2 days vs 16.3 days, p=0.263). Secondary endpoints evaluating opioid doses 24 hours prior to starting ketamine versus the first 24 hours of ketamine use (1905.2 mg vs 1339.9 mg, p=0.014) as well as the opioid doses 24 hours prior to starting ketamine versus 24 hours after stopping ketamine (1905.2 mg vs 1140.4 mg, p=0.0084) were both significantly lower in patients after receiving ketamine. All opioid doses are reported in oral morphine equivalents. Time to next admission versus time since previous discharge was numerically longer after the admission where patients received ketamine, but this did not reach statistical significance (45.7 days versus 40.3 days, p=0.59).
Conclusions: This study has shown that low-dose ketamine infusions are effective at decreasing opioid usage in addition to standard pain regimens for adult patients admitted for VOC pain.
Submitting Author: Jennifer Froomkin
Organization: University of Chicago Medicine
Authors: Jennifer Froomkin, Pharm.D.; Randall Knoebel, Pharm.D., BCOP; David Dickerson, M.D.; Hailey Soni, Pharm.D., BCPS; Angela Kerins, Pharm.D., BCPS; Jennifer Austin, Pharm.D., BCPS University of Chicago Medicine, Chicago, Illinois
*********************************************************************
5. ICHP Poster Presentations – Original
(Presented as Poster #5)
Title: Time Within Therapeutic Range: A Comparison of Three Tacrolimus Formulations in Renal Transplant Recipients
Abstract:
Purpose: To compare three formulations of
tacrolimus (TAC) and assess differences in time within therapeutic range (TTR)
and variability in levels.
Methods: Renal transplant recipients from 01/01/2013 to 10/01/2017 were retrospectively identified for analysis. Deviation from standard TAC protocol or formulation changes excluded patients. The primary outcome is percentage in TTR (%TTR) over the first 12 weeks post-transplant. Secondary outcomes include: TAC CV%, TAC levels, TAC dose, eGFR, rejection, and patient/graft survival.
Results: Research in Progress
Conclusions: Research in Progress
Submitting Author: Karen Khalil
Organization: University of Illinois Hospital & Health Sciences System
Authors: Karen A. Khalil, PharmD, PGY2 Solid Organ Transplant Pharmacy Resident; Patricia M. West-Thielke, PharmD, Director of Clinical Transplant Research; Alicia Lichvar, PharmD, MSCR, BCPS, Transplant Clinical Pharmacist; Enrico Benedetti, MD, FACS, Warren H. Cole Chair in Surgery, Professor and Head of the Department of Surgery; Shree Patel, PharmD, BCPS, Transplant Clinical Pharmacist All authors affiliated with and employed at University of Illinois Hospital & Health Sciences System.
*********************************************************************
6. ICHP Poster Presentations – Original
Winner: Best Original Poster Presentation 2018
(Presented as Poster #6)
Title: Impact of psychological debriefing on the mental health of pharmacy residents participating in a 24-hour, in-house clinical pharmacy on-call program
Abstract:
Purpose: The clinical pharmacy on-call program
at the University of Chicago Medicine is a 24-hour, in-house service provided
by pharmacy residents. The on-call pharmacy resident responds to a variety of
emergent clinical scenarios involving adult and pediatric patients. During
these shifts the pharmacy resident may experience unfortunate or unanticipated
patient outcomes which may lead to higher rates of stress, anxiety, and
depression. While higher rates of depression in medical students and residents
are well established, there are few studies describing mental health in
pharmacy residents. This study aims to assess the impact of a 24-hour,
in-house, on-call clinical pharmacy program on pharmacy resident mental health
and the effects from the implementation of a formalized psychological
debriefing process on stress, anxiety, and depression.
Methods: From June 2017 to June 2018, ten PGY1 pharmacy residents will be evaluated as they participate in a 24-hour, in-house, on-call clinical pharmacy program. Immediately after each shift, pharmacy residents will complete a modified Depression Anxiety Stress Scale (mDASS-21) during their first paired introductory shift, first five independent shifts, midpoint shift, and final shift. The severity of stress, anxiety, and depression will be measured as normal, mild, moderate, severe, or extremely severe. Immediately following the completion of the mDASS-21, pharmacy residents will undergo psychological debriefing with a pharmacy preceptor to discuss difficult situations and their emotional state during their shift. Compiling information from the mDASS-21 and debrief, pharmacy residents will be evaluated to assess resident mental health during the on-call shift. Statistical analysis will be performed using descriptive and univariate inferential statistics.
Results: Research in progress.
Conclusions: Research in progress.
Submitting Author: Kevin Mercer
Organization: University of Chicago Medicine
Authors: Kevin Mercer, PharmD, PGY1 Pharmacy Resident, University of Chicago Medicine; Sajni Patel, PharmD, BCPS, Cardiology Clinical Pharmacy Specialist, University of Chicago Medicine; Samantha Bastow, PharmD, BCPS, Clinical Pharmacy Manager, University of Chicago Medicine; Randall Knoebel, PharmD, BCOP, PGY1 Pharmacy Residency Program Director, University of Chicago Medicine; Hailey Soni, PharmD, BCPS, Internal Medicine Clinical Pharmacy Specialist, University of Chicago Medicine; Laura Lourenço, PharmD, BCPS, Solid Organ Transplant Clinical Pharmacy Specialist, University of Chicago Medicine; Jennifer Austin Szwak, PharmD, BCPS, Internal Medicine Clinical Pharmacy Specialist, University of Chicago Medicine
*********************************************************************
7. ICHP Poster Presentations – Original
(Presented as Poster #7)
Title: Impact of a PharmD consult to improve futile medication discontinuation rates in a home hospice program
Abstract:
Purpose: The objective of this study is to
determine whether speaking with a hospice pharmacist about futile medications
will improve discontinuation rates when compared with speaking with the hospice
nurse. Many patients admitted to hospice
have polypharmacy, many of which are deemed medically unnecessary. However, there is currently no existing data
surrounding the benefit of a pharmacist in the hospice setting in terms of
futile medication discontinuation rates / deprescribing. Once the patient is admitted to hospice
services, these futile medications may be stopped and replaced with comfort
medications. Some of these futile meds have the potential to add to the pill
burden and decrease quality of life for a multitude of patients. Common futile medications include maintenance
chronic obstructive pulmonary disorder (COPD) inhalers, anticoagulants,
vitamins and minerals, anti-hyperlipidemia agents, and acetylcholinesterase
inhibitors. The Centers for Medicare and Medicaid Services stipulates all
medications must be identified as related or unrelated to the terminal
prognosis, and a determination made whether hospice or a third-party payer will
cover their cost, often placing unnecessary financial burden on both patients
and hospice organizations. Recently a
new pharmacist clinical service was launched within our hospice to address the
difficulty in futile med discontinuation experienced by the nursing staff. Evaluation of the hospice pharmacist
consultation service in discontinuing futile medications is the next phase of
the project.
Methods: All patients referred to Hospice of Southern Illinois with an identified futile medication will be randomly assigned to usual medical care (nursing visits) or a PharmD consult where the pharmacist will discuss harms versus realistic benefits of the futile medication with the patient and/or caregiver. After IRB approval and once the study period has ended, the discontinuation rates of these futile medications between a PharmD consult and usual care will be compared. If a patient and/or caregiver requests to speak to the pharmacist, this patient will be counted as a non-discontinuation by the nurse and will not be assigned to either one of the intervention groups.
Results: Research in Progress
Conclusions: Research in Progress
Submitting Author: Amanda Daniels
Organization: Hospice of Southern Illinois
Authors: Amanda M Daniels, PharmD, PGY2 Pain and Palliative Care Pharmacy Resident, Hospice of Southern Illinois Ellen Middendorf, MD, Medical Director, Hospice of Southern Illinois Christopher M Herndon, PharmD, Professor, SIUE School of Pharmacy
*********************************************************************
8. ICHP Poster Presentations – Original
(Presented as Poster #8)
Title: Retrospective institutional assessment of vancomycin dosing protocol on the attainment of goal serum trough concentrations in the pediatric setting
Abstract:
Purpose: The primary objective of this study is
to determine the clinical efficacy of our institution’s vancomycin collaborative
practice agreement on goal serum trough concentrations in the pediatric
population. Currently, patients receive starting doses of 20
milligrams/kilogram/dose every eight hours. Previous studies have shown this
dose acheived goal trough concentrations (10 -20 micrograms/liter) in 37% of
patients. Secondary analyses will be conducted to examine vancomycin dosing
variations in age groups and BMI-for-age percentiles. Currently, there is
little evidence regarding effective doses in these subgroups. This study will
help optimize vancomycin dosing in several patient populations.
Methods: This study will be submitted to the Institutional Review Board for approval under exempt status, and will be a retrospective cohort analysis of patient electronic medical records from a children’s hospital in central Illinois. Data will be collected from September 1, 2012 to September 1, 2017. Patients will be included if they were between the ages of 31 days and 18 years, received vancomycin per pharmacy dosing recommendations, and had at least one serum trough concentration recorded at the time of the encounter. Patients will be excluded if they were in the NICU at time of trough draw, received vancomycin for procedural prophylaxis, or received doses recommended by non-pharmacy personnel. The primary outcome will be dose at target trough concentration, which will be determined by comparing the initial dose of vancomycin to the dose that obtained goal trough levels. Secondary outcomes will include the number of dose changes to obtain target trough, time to target trough, duration of vancomycin exposure, time to de-escalation/discontinuation, frequency of dosing, frequency of trough achievement with 1st dose, serum creatinine and urine output changes, frequency of acute kidney injury under dosing protocol, and number of held doses or discontinuation due to adverse effects.
Results: Research in progress
Conclusions: Research in progress
Submitting Author: Ashley Walter
Organization: OSF Healthcare St. Francis Medical Center
Authors: Ashley Nicole Walter PGY-1 Pharmacy Resident - OSF Healthcare St. Francis Medical Center and Children's Hospital of Illinois Doctor of Pharmacy - Butler University Bachelor of Science - Purdue University
*********************************************************************
9. ICHP Poster Presentations – Original
(Presented as Poster #12)
Title: Evaluation of Outpatient Infusion Medication Safety Monitoring at a Community Teaching Hospital
Abstract:
Purpose:
To evaluate the appropriateness of safety monitoring for medications
administered at an outpatient infusion clinic after implementing pre-infusion
checklists.
Methods: This study is a prospective analysis of laboratory monitoring obtained from newly implemented pre-infusion checklists from subjects receiving high-risk medications at the outpatient infusion clinic. Patients with a scheduled appointment at the infusion clinic to receive abatacept, tocilizumab, vedolizumab, infliximab, zoledronic acid, or rituximab were included in the study. A pharmacist designed checklist was created for each high-risk medication commonly administered at the outpatient infusion clinic. Pre-infusion checklists included recommended safety monitoring such as Hepatitis B surface Antigen (HBsAg) test, tuberculosis (TB) test, liver function test (LFT), complete blood count (CBC), and up-to-date vaccinations. Nursing in-services were given at the outpatient infusion center to introduce the staff to the pre-infusion checklists and encourage their compliance. Once implemented, the infusion clinic nurses completed the pre-infusion checklists approximately 1-2 days before patient’s scheduled appointment. The pharmacist reviewed all checklists and lab results to determine compliance with recommended monitoring, contacted providers to obtain missing labs, and coordinated with providers to order lab tests prior to initial and maintenance therapy. The pharmacist communicated to the infusion clinic nurse if an intervention was needed prior to the patient’s scheduled infusion. The primary objective of this study was to identify the percentage of medications that were appropriate to dispense before the checklists were implemented compared to the percentage of medications that were appropriate to dispense after the checklist was implemented.
Results: Research in Progress
Conclusions: Research in Progress
Submitting Author: Crystal Dedes
Organization: Advocate Illinois Masonic Medical Center
Authors: Ryan Sayler, PharmD, BCPS Clinical Pharmacist at Advocate IL Masonic Medical Center Crystal Anna Dedes, PharmD, PGY-1 Pharmacy Practice Resident at Advocate IL Masonic Medical Center
*********************************************************************
10. ICHP Poster Presentations – Original
(Presented as Poster #13)
Title: Implementation of a Pharmacist-Directed Medication Therapy Management Service for Oral Chemotherapy Patients at an Outpatient Cancer Center
Abstract:
Purpose: The purpose of this study is to
develop, implement, and evaluate the impact of pharmacist involvement in
patients receiving oral chemotherapy with respect to drug-interactions, dosing
adjustments based on laboratory parameters and patient adherence.
Methods: Electronic health records will be used to conduct a retrospective chart review of all oral chemotherapy prescriptions prescribed by oncology physicians at the Creticos Cancer Center from January 1, 2017 through March 31, 2017. Additionally, a prospective review of adult patients prescribed oral chemotherapy will be conducted from December 15, 2017 through March 15, 2018. All prescriptions will be evaluated for proper indication, dosing, drug-drug interactions, pertinent laboratory parameters, appropriate adjustments based on toxicities, as well as documentation for adherence. Problems discovered will be reported to the physician for review. Patients receiving oral hormonal chemotherapy will be excluded from the study. Pharmacist interventions that led to a change in home medications or chemotherapy treatment will be documented. One week following initiation of oral chemotherapy, the pharmacist will follow-up with a phone call to the patient. The patient will be interviewed about indication, dosing, side effects, methods of adherence, and any barriers to treatment. During this call, the patient will receive medication counseling from a pharmacist addressing all pertinent information about their prescription. The patient will be contacted again 28-days after the start of therapy to re-assess understanding of their medication post-pharmacist counseling. Additional information regarding pharmacist time commitment will be collected. Data comparison of historical data versus post–implementation data will be analyzed focusing on the overall impact of pharmacist intervention and patient adherence.
Results: Research in Progress
Conclusions: Research in Progress
Submitting Author: Niree Kalfayan
Organization: Advocate Illinois Masonic Medical Center
Authors: MyChau Nguyen, PharmD - University of Illinois College of Pharmacy, BCOP – Oncology Pharmacist, Advocate IL Masonic Medical Center Niree Kalfayan, PharmD - Midwestern University, Chicago College of Pharmacy, PGY-1 Pharmacy Practice Resident, Advocate IL Masonic Medical Center
*********************************************************************
1. ICHP Poster Presentation – Encore
(Presented as Poster #9)
Title: Use of a dashboard for monitoring clinical pharmacy services in a large medical center
Abstract:
Purpose: A dashboard is a management tool that
provides a quick, visual picture with graphic displays or tables depicting
select measures and is representative of the functioning of a business entity
or division over a period of time. To
qualify for use in a dashboard, items being measured should be easy to measure
and obtain, reflect desired goals, account for fluctuations in populations, can
clearly show current status or trends, and allow for predictions. The purpose
of this report is to describe the use of a dashboard used to monitor and set
goals for specific clinical services in a large medical center pharmacy
department.
Methods: Fourteen measures of clinical pharmacy services in a 616-bed medical center were chosen based on desired hospital improvement targets. Four assessed elements of antimicrobial stewardship (interventions resulting from Emergency Department culture reviews, vancomycin discontinuation in patients with culture-negative neutropenia, antibiotic discontinuation on medical/surgical care unit, and reducing overall usage of piperacillin/tazobactam) and 4 measures assessed patient medication education (renal transplant patients, patients starting warfarin, any patient on anticoagulants, and any education). The remaining were measures of critical International Normalize Ratio values resulting from pharmacy dosing service, analgesia and sedation medication reviews and pro-active interventions, defects associated with admission reconciliation for renal transplant patients, elimination of inappropriate stress ulcer prophylaxis on intermediate and acute neurology units, and parenteral antihypertensive drug selection. Four dashboard items were measured hospital-wide, three focused on the critical care units, two for renal service and the remaining 5 were unit-specific. Target goals for each dashboard measure were based on desired performance and past activity. Fifteen monthly measurements for the 14 activities, adjusted for patient census, were recorded from October 2015 to December 2016. To provide a quick visual status and trend analysis, each monthly box was colored green, yellow or red representing either the specific measure is meeting or exceeding the goal, or is within about 10-20% of the goal, or exceeds 20% of the targeted goal, respectively.
Results: Over the 15 month period, seven of the 14 clinical measures showed no change, 6 met or exceeded their goals, and one showed a trend away from the goal. Two measures essentially met or exceeded theirs goals (All med education and critical INRs) and two failed to meet their goals (SUP discontinuation and antihypertensive selection) for the entire study period. Out of the 189 total monthly measurements, 85 month scores met or exceeded the desired goal (Green), 66 scores did not meet the goals by more than 20% (Red), and 38 scores were within 10% of the desired goals (Yellow). Factors that may have influenced the outcomes include overall workload volume, lower prioritization of these services, and pharmacist limitations in influencing specific outcomes. Review of goals and changes should be considered for measures showing little or no change.
Conclusions: Dashboards provide a unique method of displaying and evaluating the status of a pharmacy’s clinical services provided throughout a medical center. This 15-month report displays 14 selected measures that were used by hospital and pharmacy management, including pharmacy staff, to set desired clinical goals and to quickly assess their status over time.
*********************************************************************
2. ICHP Poster Presentation – Encore
(Presented as Poster #10)
Title: Improving compliance with inpatient utilization of medications that have Elements to Assure Safe Use at a large academic medical center
Abstract:
Purpose: To create a standardized compliance
process for medications that have Elements to Assure Safe Use (ETASU) at
Northwestern Memorial Hospital (NMH).
Methods: The Food and Drug Administration (FDA) website was reviewed in September 2017 to determine which medications on the NMH formulary had ETASU requirements. Further evaluation of the ETASU requirements for the targeted NMH formulary medications was conducted to determine which had relevant inpatient requirements. Internal electronic resources were reviewed to determine what ETASU requirements were already embedded into the medication ordering and verification process. Company REMS programs were also called to verify inpatient ETASU pharmacy requirements.
Results: Review of relevant inpatient ETASU requirements resulted in identification of four formulary medications that warranted modifications to the current institutional process. Uniform actions to improve compliance with ETASU standards were proposed for order entry, pharmacist verification, electronic documentation, and staff education.
Conclusions: A standardized process was created for pharmacists to assess and document ETASU medication requirements within the electronic medical record that will generate improved compliance with ETASU regulations resulting in enhanced patient care for these selected medications.
Submitting Author: Brittany Lee
Organization: Northwestern Memorial Hospital
Authors: Brittany Lee, PharmD Northwestern Memorial Hospital PGY-1 Pharmacy Practice Resident Gerald Elliott, PharmD Northwestern Memorial Hospital PGY-1 Pharmacy Practice Resident Elizabeth Short, PharmD, BCPS, BCCCP Northwestern Memorial Hospital Practice Coordinator, Clinical Services (non-oncology)
*********************************************************************
3. ICHP Poster Presentation – Encore
(Presented as Poster #11)
Title: Implementation of phone etiquette and triage training in pharmacy technicians at a large academic medical center
Abstract:
Purpose: To implement phone etiquette and
triaging techniques into pharmacy technician training to improve nurse,
pharmacist, and pharmacy technician satisfaction with phone calls to the
Pharmacy Department
Methods: A survey was sent out to pharmacists, pharmacy technicians, and nurses to evaluate the current satisfaction with pharmacy phone etiquette and triaging. A phone etiquette and triaging training program and competency checklist were created and administered to pharmacy technicians working in the central pharmacy at Northwestern Memorial Hospital. A follow-up survey was sent post phone training completion to determine if there were any changes in satisfaction or competency
Results: There were 274 responses to the pre-training survey and 60 responses to the post-training survey. Satisfaction with phone calls to the pharmacy showed improvement after phone call triage training was implemented in regards to: Time assessment Professionalism Competence Overall satisfaction The majority of responders to the first survey were nurses working day shift (67%). The proportion of responders to the second survey was approximately 1/3 each of nurses, pharmacists, and pharmacy techs.
Conclusions: Given the positive results and feedback, we plan to implement a formal phone triaging and etiquette training and competency into pharmacy technician training for both current and future employees in all pharmacy satellites. We also plan to send out a second post-training survey once all technicians have been trained and sufficient time has been passed to determine if improvement is noticed in competence and etiquette.
Submitting Author: Tyler Alverson
Organization: Northwestern Memorial Hospital
Authors: Tyler Alexsandra Alverson, PharmD, PGY1 Resident, NMH Joanna Urban, PharmD, PGY1 Resident, NMH Ana Fernandez, CPhT, Pharmacy Practice Coordinator, NMH Noelle RM Chapman, PharmD, Pharmacy Manager/PGY1 Residency Program Director, NMH
*********************************************************************
4. ICHP Poster Presentation – Encore
(Presented as Poster #14)
Title: Active Pharmacy Student Engagement in Inter-Professional Education
Abstract:
Purpose: Pharmacy students are consistently
taught about the importance of patient teaching in order to minimize misuse of
medications and to improve awareness and management of selected side effects
associated with drug therapy. The evaluation of inter-professional learning has
been examined in multiple studies finding an increase in patient baseline
knowledge and improving collaboration in the work force. This project was
designed to identify the strengths and weaknesses of inter-professional
medication teaching conducted by fourth year pharmacy students for their
“patients” who were first year physician assistant students.
Methods: During orientation week, PA students begin a week-long learning experience designed to introduce concepts about medication adherence and collaborative practice. PA students receive a ‘prescription’ from a ‘provider,’ with a written note explaining to the student, in the role of “patient,” that he/she is to start (a) medication(s) based on recent lab work. As “patients,” the PA students, were purposely told little about their condition(s), prescribed medication(s) or clinical purpose(s). Patients not understanding their condition(s) or medication(s) is a common theme in pharmacy practice. The PA student “patients” took their ‘prescription(s)’ to advanced practice student pharmacy students, acting as “pharmacists,” to be ‘filled.’ Pharmacy students counsel their “patients” about the newly prescribed medication(s), common indications, what to expect, when to discontinue or when to contact the prescriber. The pharmacy students also answered questions from their “patients.” The PA students were instructed to follow “pharmacists” recommendations and to continue to take their medication(s) until they returned to their provider for follow-up. A survey designed to assess compliance and identify medication understanding was sent to all students, with separate versions for each discipline. 3.
Results: This activity was well received by all students. Three fourth year pharmacy and thirty physician assistant students (100%) participated in this experience. There were eighteen questions specific to each discipline as well as a common survey. All questions were original to the activity. We found that 80% of the PA orientation students had never participated in an active inter-professional activity. We also found favorable results (75% Approval) for collaboration and understanding of the role of other healthcare professionals. The activity did, however, identify a few, yet important, gaps in medication information communicated by the pharmacy students to their “patients.” Pharmacy students, in general, provide too much information (47%), and tend to offer boiler plate rather than individualized advice (70%). We found that one student often (50%) omitted critical information about the medications, and also neglected to introduce her/himself. Most students (75%) indicated this activity was a useful and important learning activity. Just about 50% of students found it difficult to adhere for 2 weeks to a simple medication schedule involving 2 medications each taken no more often than twice a day. 4.
Conclusions: We plan to continue to update this activity by making changes per the survey results, increasing the number of pharmacy student involvement, incorporating an inter-professional reading prior to the start of the activity and a debrief at the end of the activity.
Submitting Author: Jorie Kreitman
Organization: Rosalind Franklin University of Medicine and Science
Authors: Jorie F Kreitman- PharmD Candidate 2018 Rosalind Franklin University of Medicine and Science Michael A Fotis, RPh - Northwestern University Feinberg School of Medicine-Faculty Department of Medical Education, Physician Assistant Program Kristine M Healy MPH, PA-C Northwestern University Feinberg School of Medicine -Associate Director Physician Assistant Program, Assistant Professor - Medical Education
*********************************************************************
5. ICHP Poster Presentation – Encore
(Presented as Poster #15)
Title: Identifying Barriers to Performance of a Newly Implemented Spontaneous Awakening Trial Protocol
Abstract:
Purpose: The purpose of this study was to
evaluate compliance with the newly implemented SAT paired protocol and identify
barriers in daily performance.
Methods: We conducted a retrospective cohort study of patients admitted to the medical ICU (MICU) that required mechanical ventilation for at least 24 hours to assess compliance with the paired SAT/SBT protocol. SBTs were routinely completed and documented by respiratory care providers prior to implementation of the SAT screening and performance process so our analysis focused on the SAT portion of the process. Bedside nurses caring for the patient conducted a safety screen to rule out the presence of agitation, seizures, substance withdrawal, use of paralytics, active myocardial ischemia, or elevations in intracranial pressure. If all criteria were deemed to be absent, the nurse discontinued all continuous sedatives except those necessary for pain control. Patients were deemed to have failed the SAT if any of the following were present in the 4 hours following SAT initiation: agitation, tachypnea, respiratory distress, hypoxia, or cardiac arrhythmia. If a patient developed any of the SAT failure criteria, sedatives and analgesics were re-initiated at 50% of the prior infusion rate. Patients that did not develop these criteria in the 4 hour period following the screen were deemed to have passed the SAT. We identified steps which were not completed within the SAT process and categorized them as one of the following potential barriers: missing SAT orders, SAT safety checklist not completed, SAT safety screen not completed accurately, SAT safety screen failure met, failure to perform SAT, failure to document SAT, or SAT failure criteria met. Data is presented using appropriate descriptive measures including n (%) or mean (+/- standard deviation).
Results: SATs were ordered in 25% of total days evaluated. When SAT orders were placed, SAT safety checklists were completed in 50.6% of patients. These safety checklists were completed accurately 81.6% of the time. Regardless of SAT order presence, SATs were performed in 65.1% of total days evaluated. As expected, patients who met failure criteria (12%) within the safety checklist did not receive a SAT. Of the SATs performed, only 14.2% of them were documented.
Conclusions: Major areas of noncompliance are present in our current protocol with the largest being patients missing SAT orders. Without these orders, there is no prompt to ensure accurate screening, performance and documentation of SATs. Future directions include thorough reeducation of healthcare personnel, primarily physicians and pharmacists, to stress importance of SAT orders and potential order set modifications including an automatic order within existing sedation order sets.
Submitting Author: David Antoine
Organization: Northwestern Memorial Hospital
Authors: DA Antoine Pharm.D, Northwestern Memorial Hospital, CT Makowski Pharm.D, Northwestern Memorial Hospital, AT Ammar Pharm.D, BCPS, BCCCP, Northwestern Memorial Hospital, CJ Cooper, Pharm.D, BCPS, BCCCP, Northwestern Memorial Hospital, BD Lizza, Pharm.D, M.S., BCPS, BCCCP, Northwestern Memorial Hospital
*********************************************************************
6. ICHP Poster Presentation – Encore
Winner: Best Encore Poster Presentation 2018
(Presented as Poster #16)
Title: Evaluation of standardized drug concentrations for intravenous adult continuous infusions
Abstract:
Purpose: American Society of Health-System
Pharmacists (ASHP) in conjunction with the FDA is leading the Standardize 4
Safety initiative, a method to prevent patient harm and death from medication
errors. The goal of Standardize 4 Safety is to implement standardized
concentrations and dosing units for various dosage forms as recommended on the
ASHP website. Northwestern Memorial
Hospital (NMH) supports Standardize 4 Safety and the goal of this project is to
compare current NMH adult continuous infusion concentrations to ASHP’s
recommended concentrations and analyze the potential impact of adopting their
recommendations.
Methods: Study Design: retrospective, clinical, operational and cost analysis. A gap analysis was performed on adult IV infusion concentrations used at NMH to determine how they vary from the ASHP-recommended concentrations. The IV medications with variations to be studied include: amiodarone, bumetanide, dobutamine, dopamine, epinephrine, labetalol, nicardipine, nitroglycerin, norepinephrine, phenylephrine and rocuronium. Data was collected from August 1st, 2016 to August 1st, 2017 on the total number of infusion bags dispensed per patient order, start and stop times for the IV medication, and the dose or rate. From this information, the number of bags dispensed per year for each IV adult infusion was calculated based on current NMH concentrations. The number of infusion bags needed with the new ASHP recommended concentrations to dispense the same total dose per year was predicted. The difference in number of bags dispensed and change in volume the patient would receive with ASHP recommended concentrations was calculated. The annual drug acquisition cost difference was calculated based off of commercially available products compared to compounded products and medication preparation time. Administration and nursing workflow changes and training was also evaluated to assess the impact on hospital operations.
Results: If all analyzed ASHP recommended concentrations were adopted: 33% would result in cost savings. 54% would decrease the number of bags dispensed per day allowing for fewer interruptions in therapy, less burden on nursing workflow, and fewer dispenses for pharmacy staff. 62% would result in a decrease in infusion volume. 55.6% of ASHP recommended concentrations included in this analysis are commercially available. Commercially available products provide longer stability than compounded medications, thus decreasing waste and pharmacy compounding requirements. Adopting the ASHP recommended concentrations would not cause a change in peripheral versus central administration except for the increased concentrations recommended for amiodarone and phenylephrine, where only central administration is recommended.
Conclusion: Based on this analysis, we would recommend considering adoption of ASHP’s recommended concentrations for amiodarone, dobutamine, dopamine, epinephrine, labetalol, and phenylephrine. If we adopted these, the Total Net Cost Difference: - $768 per year and Total Net Dispense Difference: -6.6 bags per day.
Submitting Author: Brittany Huff
Organization: Northwestern Memorial Hospital
Authors: Sarah Schaidle, PharmD, Brittany Huff, PharmD, Kelsea Caruso, PharmD, Katie Gauen, PharmD, Lara Ellinger, PharmD, BCPS Northwestern Memorial Hos
*********************************************************************
7. ICHP Poster Presentation – Encore
(Presented as Poster #17)
Title: Implementation and evaluation of an error and complaint reporting system in a specialty and ambulatory care pharmacy
Abstract:
Purpose: To assess areas for improvement within
the pharmacy and then focusing to improve the error and complaint reporting
system by restructuring the process to create a workflow that effectively
evaluates and addresses the problems within the specialty pharmacy
Methods: The objective of the project was completed by identifying the highest need for intervention based on the FY-2017 error and complaint reports, implementing a new method for collecting these error and complaint reports, then analyzing the process and comparing it with the pre- implementation data. Evaluation of the quality procedures and risk management within the pharmacy was done by reviewing the error reporting and URAC standards. Implementation of a new method for collecting the errors and complaint forms was be based on feedback from the staff, surveys were conducted asking about the barriers and issues to completing the complaint and error reports. The responses guided the development of the implementation of a different workflow for reporting errors. The data was analyzed and guided the development of the implementation of different workflow for reporting errors.
Results: Within the 216 total ISMP objectives, 15% were non-compliant and 47% of those were within the Quality Procedures & Risk Management category. The specialty pharmacy has approximately 64% fully implemented activities to all patients, prescriptions, drugs, or staff within the quality processes and risk management characteristic. Biggest barriers to staff reporting include time constraints and lack of understanding of “what” to report. The total number of complaint reports for the 2017 fiscal year was sixty averaging five reports per month. There were three months that did not have any incidences reported. Of the complaints reported, 22% were due to shipping, 28% due to delivery, and 20% due to incorrect processing
Conclusions: Both the quality and quantity of the complaints and error reports were lower than expected for the fiscal year. Implementing a new workflow system to better collect and analyze this data will overall improve the quality and safety for patients.
Submitting Author: Bridget Dolan
Organization: Northwestern Memorial Hospital
Authors: Kayla Hoogendoorn PharmD, MPH, Northwestern Memorial Hospital Bridget Dolan PharmD; Northwestern Memorial Hospital Nadine Isho, PharmD; Northwestern Medicine Specialty Pharmacy
*********************************************************************
8. ICHP Poster Presentation – Encore
(Presented as Poster #18)
Title: Feasibility and impact of bortezomib batching in a high-volume outpatient oncology clinic
Abstract:
Purpose: To evaluate the impact of preparation
of bortezomib doses in advance (batching doses) on patient throughput as well
as nursing and patient satisfaction
Methods: Evaluate current bortezomib volume and bortezomib administration trends -Prepare bortezomib doses as a single batch in advance on the morning of expected administration -Evaluate time from order verification to drug administration compared to historical controls prior to bortezomib batching -To determine appointment duration -Determine waste and financial implications
Results: Time from pharmacist verification to nursing administration was used as a surrogate endpoint ~10 minutes were saved on average per patient in intervention arm Bortezomib appointments have since been decreased from 90 minutes to 60 minutes.
Conclusions: With this change, we estimate on average: ~54 patients per week; 81 hours of chair time per week; Gained chair time 27 hours per week. ~ 5 hrs per day (Figure 3) Approximately 5% of bortezomib was wasted during batching intervention compared to 1% waste in the control arm. Future Directions Evaluation of nursing workflow Limitation of study: no direct measurement from time of pharmacy dispense to nursing administration Evaluate nursing processes to determine delays in administration Survey of patients and nurses to evaluate satisfaction with more efficient pharmacy preparation of doses Potential for incorporation of extended stability of bortezomib to batched doses Batching of other medications in oncology clinic
Submitting Author: Alison Svoboda
Organization: Northwestern Memorial Hospital
Authors: Amanda Finch, PharmD, Alison Svoboda, PharmD, Farah Barada, PharmD Northwestern Memorial Hospital
*********************************************************************
1. ICHP Poster Presentation – Student
(Not presented)
Title: Amoxicillin dosing frequency in pediatric patients diagnosed with community acquired pneumonia and its effect on hospital readmission rates.
*********************************************************************
2. ICHP Poster Presentation – Student
Winner: Best Student Poster Presentation 2018
(Presented as Poster #20)
Title: Pneumococcal Vaccination in Pediatric Cystic Fibrosis Patients
Abstract:
Purpose: The purpose of this quality
improvement project is to provide pediatric cystic fibrosis (CF) patients with
Pneumovax23 (PPSV23) and when appropriate, completion of their Prevnar (PCV13)
vaccination series at Cardinal Glennon Children’s Hospital CF Clinic. There is
currently a lack of administration of Pneumovax in this patient population.
This project is also designed to educate the CF clinic physician team regarding
the following information. Patients with CF are generally at higher risk of
contracting pneumococcal infection and are candidate for vaccination with
Pneumovax due to the comorbidities associated with their condition. Pneumovax
is approved for use in at risk patients aged 24 months and older. Patients at
increased risk include those with a history of chronic lung disease. The
definition of chronic lung disease for this benefit group includes those with
asthma that have been treated with a long term course of corticosteroids as
well as patients with chronic obstructive pulmonary disease and emphysema. This
information may be reasonably extrapolated this patient population as many of
these patients have a concurrent history of asthma and have been treated with
long term courses of inhaled or oral corticosteroids. Inclusion criteria
includes diagnosis of CF, status as a current CF clinic patient and age greater
than two years old. Patients aged two to twenty-one years old were included in
this project.
Methods: Data will be collected through review of the patient’s electronic medical record, the Cystic Fibrosis Foundation patient registry and other immunization reporting databases, when possible. Consultation with physician’s offices beginning in late January will also be used to assess any previous Pneumovax administration and pertinent vaccination history. The CF team physicians will be given education regarding indications and guidelines for PPSV23 administration in pediatric patients greater than two years old, as well as indications for PCV13 catch up vaccinations. After time for implementation of vaccination protocol in eligible patients a review of vaccination history will be completed to confirm if vaccination was given. IRB approval was granted through Cardinal Glennon Children’s Hospital and Southern Illinois University Edwardsville School of Pharmacy, through collaborative agreement.
Results: Research in Progress
Conclusions: Research in Progress
Submitting Author: Devin Dinora
Organization: Southern Illinois University Edwardsville - School of Pharmacy
Authors: Devin R Dinora, Pharm.D. Candidate, SIUE School of Pharmacy Lisa Lubsch, PharmD, BCPPS, AE-C, SIUE School of Pharmacy, SSM Cardinal Glennon Children's Hospital

Government Affairs Report
The Beat Goes On!
by Jim Owens and Scott Meyers
The second major deadline has passed in the Spring Session of the Illinois General Assembly and the pace of activity has picked up a little. The deadline in question occurred on April 13th when substantive bills needed to move out of their respective committees in Springfield and on to 2nd Reading or be referred back to the Rules and Assignment Committees of the House and Senate. While a handful of bills were given extensions for more discussion at the committee level, we did see significant movement and amendments to some of the bills we have been monitoring and working on for the first three months of this session.
HB0274 – Michelle Mussman, Schaumburg, D – The pharmacist hormonal birth control prescribing bill came out of the Health Care Licenses Committee with the support of the OB/GYN doctors and a neutral stance by the Illinois State Medical Society (ISMS). The only concerns lie with the Department of Healthcare and Family Services (DHFS) with regard to payment for the cognitive services that will accompany the actual dispensing. We hope that more compromise can be accomplished but this is a great step in the right direction!
HB4707 – Sue Scherer, Decatur, D – This bill initially intended to make hydrocodone a schedule I controlled substance in Illinois but a variety of health care associations expressed serious concerns for patient care and the sponsor amended the bill removing that change and in its place established the Prescription Drug Task Force made up of legislators, law enforcement representatives and representatives from various health care associations including ICHP. The bill is now on 2nd Reading in the House.
HB4907 - Mike McAuliffe, Chicago, R – Intended to require HIPAA training for prescriber and dispenser PMP designees has been amended to allow only licensed pharmacy personnel to be designated access to the PMP by pharmacists and therefore removes any concerns ICHP and IPhA had with its initial language. This bill also adds one dentist to the PMP Peer Review Subcommittee of the PMP Advisory Committee. The bill has been placed on 2nd Reading in the House as amended.
HB5442 – Jim Durkin, Burr Ridge, R – Would have amended the Controlled Substance Act to require all hospitals to post all administered doses of controlled substance to the PMP but has now been tabled. ICHP, IPhA and IRMA strongly opposed this bill.
HB5602 – Jonathan Carroll, Buffalo Grove, D – This bill removes the requirement of U.S. citizenship or legal immigration status as criteria for obtaining a pharmacist license. This bill is on 2nd Reading in the House and ICHP has taken a neutral position on it.
SB2524 – Chapin Rose, Champaign, R – Amends the Environmental Protection Act by creating the Pharmaceutical Disposal Task Force. The Task Force will coordinate statewide efforts regarding proper disposal of unused medications. This bill has passed out of the Senate and awaits committee assignment in the House.
SB2952 – Melinda Bush, Grayslake, D – The Senate version of HB4907 mentioned above. Placed on 2nd Reading in the Senate.
SB3170 – Steve Stadelman, Rockford, D – Provides that a prescription for medication other than controlled substances shall be valid for up to 15 months from date of issue for the purpose of refills unless the prescription state otherwise. This bill is on 3rd Reading in the Senate.
SB3431 – Sue Rezin, Peru, R – Would have restricted initial prescriptions for opiate medications to a 7-day supply for patients 18 years old and older and restrict all opiate prescriptions for patients younger than 18 to 7-day supplies. This bill has been referred back to the Assignments Committee and will probably not move further.
A list of all the bills ICHP is monitoring is provided below. If you see a bill sponsored by your State Senator or Representative, it provides a great opportunity to talk to them to find out more about their intentions and to see if you there is a way for you to provide feedback or assist in the bill’s passage. At the same time, building a better relationship with one or both of the individuals representing you in Springfield. ICHP is working for you and the profession to advance patient care and excellence in pharmacy. Look for opportunities like that to do your part!
2018 Illinois General Assembly Bill Summary
|
Bill Number |
Sponsor |
Summary |
Location |
ICHP Position |
|
SB0316 |
Steans – Chicago, D |
Amends the Illinois Clinical Laboratory and Blood Bank Act. Makes a technical change in a Section concerning the short title.
Cannabis taxation – 21 or older buy cannabis be taxed like alcohol purchase |
In Assignments
|
|
|
SB0336
Sam001
|
Harmon - Oak Park, D |
Amends SB336 by replacing everything after the enacting clause with the following: “Section 1. This Act may be referred to as the Alternatives to Opioids Act of 2018. Section 5. the Compassionate Use of Medical Cannabis Pilot Program Act is amended by changing sections 5, 10, 60, and 160 as follows…” Sam001 supporting cannabis as MAT for opioid use disorder |
Placed on Calendar Order of 3rd Reading February 27th 2018
2/22/2018
Added co-sponsor Daniel Biss
2/20/2018 |
|
|
SB1888 & HB3479 |
McCann- Jacksonville, R
Feigenholtz – Chicago, D |
Amends the Medical Assistance Article of the Illinois Public Aid Code. In addition to other specified actions required under the Code, requires a managed care community network that contracts with the Department of Healthcare and Family Services to establish, maintain, and provide a fair and reasonable reimbursement rate to pharmacy providers for pharmaceutical services, prescription drugs and drug products, and pharmacy or pharmacist-provided services. Provides that the reimbursement methodology shall not be less than the current reimbursement rate utilized by the Department for prescription and pharmacy or pharmacist-provided services and shall not be below the actual acquisition cost of the pharmacy provider. Requires a managed care community network to ensure that the pharmacy formulary used by the managed care community network and its contract providers is no more restrictive than the Department's pharmaceutical program. Effective July 1, 2018. |
Assignments in Senate Rules in House
5/5/2017
|
|
|
SB2189 |
Connelly – Napeerville, R |
Creates the Medicaid Smart Card Pilot Program Act. Requires the Director of the Department of Healthcare and Family Services to establish a Medicaid Smart Card Pilot Program to reduce the total amount of expenditures under the State's Medical Assistance Program. Provides that the pilot program shall be designed to reduce the average monthly cost under the State's Medical Assistance Program for recipients within the pilot program area by an amount that is at least sufficient to recover the cost of implementing the pilot program. Provides that the Director shall determine the geographic area to be included in the pilot program and may contract with an independent entity for the purpose of developing and implementing the pilot program. Contains provisions on required activities under the pilot program, including the distribution of Medicaid Smart Cards to designated recipients; measures the Department might take to implement the pilot program; annual evaluations; reporting requirements; extension or expansion of the pilot program; the confidentiality of health information; reports to the Inspector General; and rulemaking authority. |
Referred to Assignments Comm.
4/13/2018 |
Neutral |
|
SB2226 |
Nybo - Lombard, R |
Amends the State Police Act. Provides that a physician, physician's assistant with prescriptive authority, or advanced practice registered nurse with prescriptive authority who provides a standing order or prescription for epinephrine auto-injectors in the name of the Department of State Police shall incur no civil or professional liability, except for willful and wanton conduct, as a result of any injury or death arising from the use of an epinephrine auto-injector. Amends the Illinois Police Training Act. Provides that a physician, physician's assistant with prescriptive authority, or advanced practice registered nurse with prescriptive authority who provides a standing order or prescription for epinephrine auto-injectors in the name of a local governmental agency shall incur no civil or professional liability, except for willful and wanton conduct, as a result of any injury or death arising from the use of an epinephrine auto-injector. Makes conforming changes to the Medical Practice Act of 1987 and the Public Health Standing Orders Act. Effective immediately. |
First Reading Referred to Rules Committee in the House
2/27/2018
Chief House Sponsor Deb Conroy
2/21/2018 |
|
|
SB2334 |
Murphy - Elk Grove Village, D |
Amends the University of Illinois Hospital Act and Hospital Licensing Act. Provides that a hospital shall maintain a metal detector at each point of entry into the hospital. Provides that a hospital shall ensure that all members of the public, other than the employees of the hospital who display proper credentials, who enter the hospital at a point of entry are subjected to screening by a metal detector. Provides that individuals subject to screening shall include, but not be limited to, individuals in wheelchairs. Defines "point of entry". Effective July 1, 2018. |
Postponed - Public Health
4/11/2018 |
Neutral |
|
SB2465 |
Mulroe- Chicago, D |
Amends the Illinois Public Aid Code. Makes a technical change in a Section concerning medical services. |
Referred to Assignments
1/30/2018 |
|
|
SB2524 SAM002
|
Rose - Champaign, R |
Sen. Amendment 1: Amends the Environmental Protection Act by creating the Pharmaceutical Disposal Task Force. The task force will coordinate a statewide public information campaign to highlight the benefits and opportunities of properly disposing of pharmaceutical products. Identifies the members of the task force and responsibilities.
Sen. Amendment 2: Added representatives of physician, coroner and pharmaceutical manufacturer to the task force. |
Passed in the Senate
Sent to Rules Comm. in the House
4/17/2018 |
Neutral |
|
SB2529
|
Stadelman - Rockford, D |
Amends various acts to remove provisions allowing or requiring licensing authorities to deny, not renew, suspend, or revoke professional licenses for defaulting on an educational loan or scholarship provided by or guaranteed by a State agency. Effective immediately. |
Referred to Assignments Comm.
4/13/2018 |
Support |
|
SB2827 HB2511 |
Murphy - Elk Grove Village, D |
Amends the Medical Assistance Article of the Illinois Public Aid Code. Provides that drugs prescribed to residents of the following facilities are not subject to prior approval as a result of the 4-prescription limit: long-term care facilities as defined in the Nursing Home Care Act; community-integrated living arrangements as defined in the Community-Integrated Living Arrangements Licensure and Certification Act; and supportive living facilities as defined in the Code. |
Placed on 2nd Reading in the Senate
Added Chief Co-Sponsor
3/13/2018 |
Neutral |
|
SB2834 |
Syverson – Rockford, R |
Amends the Alcoholism and Other Drug Abuse and Dependency Act. Changes the short title of the Act to the Substance Use Disorder Act. Removes the terms "addict", "addiction", "alcoholic", "alcoholism", and "substance abuse" and their corresponding definitions. Requires the Department of Human Services to reduce the incidence of substance use disorders (rather than reduce the incidence and consequences of the abuse of alcohol and other drugs). Defines "substance use disorder". Requires the Department to design, coordinate, and fund prevention, early intervention, treatment, and other recovery support services for substance use disorders that are accessible and address the needs of at-risk individuals and their families. Requires the Department to develop a comprehensive plan on the provision of such services; assist other State agencies in developing and establishing substance use disorder services for the agencies' clients; adopt medical and clinical standards on how to determine a substance use disorder diagnosis; and other matters. Contains provisions concerning the licensing of substance use disorder treatment providers; licensure categories and services; the identification of individuals who need substance use disorder treatment using "SBIRT"; patients' rights; services for pregnant women, mothers, and criminal justice clients; and other matters. Repeals a provision of the Act establishing the Committee on Women's Alcohol and Substance Abuse Treatment. Repeals a provision of the Act setting forth the powers and duties of the Medical Advisory Committee. Makes conforming changes concerning the Substance Use Disorder Act to several Acts including the Department of Human Services Act, the Children and Family Services Act, and the Mental Health and Developmental Disabilities Administrative Act. Effective January 1, 2019. |
Placed on 3rd Reading in the Senate
4/19/2018 |
|
|
SB2849 Sam001 |
Van Pelt - Chicago, D |
Creates the Prescription Drug Repository Program Act. Requires the Department of Public Health to, by rule, establish a prescription drug repository program, under which any person may donate a prescription drug or supplies needed to administer a prescription drug for use by an individual who meets eligibility criteria specified by the Department. Sets forth requirements that prescription drugs or supplies must meet in order to be accepted and dispensed under the program. Provides that no drugs or supplies donated under the prescription drug repository program may be resold. Provides that nothing in the Act requires that a pharmacy or pharmacist participate in the prescription drug repository program. Provides for civil and criminal immunity for drug and supply manufacturers and individuals in relation to the donation, acceptance, or dispensing of prescription drugs or supplies under the prescription drug repository program. Imposes conditions on any rulemaking authority. Amends the Pharmacy Practice Act, the Wholesale Drug Distribution Licensing Act, the Senior Pharmaceutical Assistance Act, the Illinois Food, Drug and Cosmetic Act, the Illinois Controlled Substances Act, and the Cannabis and Controlled Substances Tort Claims Act to provide that persons engaged in donating or accepting, or packaging, repackaging, or labeling, prescription drugs to the extent permitted or required under the Prescription Drug Repository Program Act are exempt from provisions of those other Acts that might prohibit or otherwise regulate such activity.
Senate Amendment 001: Added clause that gave uninsured and underinsured individual priority over other eligible persons and person who is engaged in packing and relabeling of donated medication is not considered “manufacturer”. |
Referred to Assignments
4/13/2018
|
Neutral |
|
SB2889 |
Rose – Champaign, R |
Creates the Epinephrine Administration Act. Provides that a health care practitioner may prescribe epinephrine pre-filled syringes in the name of an authorized entity where allergens capable of causing anaphylaxis may be present. Provides that an authorized entity may acquire and stock a supply of undesignated epinephrine pre-filled syringes provided the undesignated epinephrine pre-filled syringes are stored in a specified location. Requires each employee, agent, or other individual of the authorized entity to complete a specified training program before using a pre-filled syringe to administer epinephrine. Provides that a trained employee, agent, or other individual of the authorized entity may either provide or administer an epinephrine pre-filled syringe to a person whom the employee, agent, or other individual believes in good faith is experiencing anaphylaxis. Provides that training under the Act shall be valid for 2 years. Requires the Department of Public Health to approve training programs, to list the approved programs on the Department's website, and to include links to training providers' websites on the Department's website. Contains provisions concerning costs, limitations, and rulemaking. Defines terms. Amends the School Code. In provisions concerning epinephrine administration, provides that epinephrine may be administered with a pre-filled syringe. Makes conforming changes. Senate Amendment 1: Changed “auto-injectors” to “injectors” Senate Amendment 2: Removes provision creating Epinephrine Administration Act. |
Placed on Calendar Order of 3rd Reading April 10, 2018 3/14/2018 |
|
|
SB2951 HB4950 |
Bush - Grayslake, D |
Creates the Early Mental Health and Addictions Treatment Act. Requires the Department of Healthcare and Family Services, and other specified agencies and entities, to develop a pilot program under which a qualifying adolescent or young adult may receive community-based mental health treatment from a youth-focused community support team for early treatment that is specifically tailored to the needs of youth and young adults in the early stages of a serious emotional disturbance or serious mental illness. Requires the Department to apply, no later than September 30, 2019, for any necessary federal waiver or State Plan amendment to implement the pilot program. Requires the Department to implement the pilot program no later than December 31, 2019 if federal approval is not necessary. Contains provisions concerning the creation of a community-based treatment model under the pilot program; the development of a pay-for-performance payment model; Department rules to implement the pilot program; and analytics and outcomes report. Requires the Department to develop an Assertive Engagement and Community-Based Clinical Treatment Pilot Program for individuals with opioid and other drug addictions. Contains provisions on in-office, in-home, and in-community services provided under the pilot program; application for a federal waiver or State Plan amendment to implement the pilot program; development of a pay-for-performance payment model; Department rules to implement the pilot program; and analytics and outcomes report. Effective immediately. |
Postponed – Human Service
3/14/2018
Added Co-Sponsor
3/14/2018 |
|
|
SB2952 HB4907 |
Bush – Grayslake, D |
Amends the Illinois Controlled Substances Act. Provides that the Department of Human Services, in consultation with the Advisory Committee, shall adopt rules allowing licensed prescribers or pharmacists who have registered to access the Prescription Monitoring Program to authorize a licensed or non-licensed designee (rather than any designee) employed in that licensed prescriber's office or licensed pharmacist's pharmacy and who has received training in the federal Health Insurance Portability and Accountability Act to consult the Prescription Monitoring Program on their behalf. Requires the Clinical Director of the Prescription Monitoring Program to select 6 members (rather than 5 members), 3 physicians, 2 pharmacists, and one dentist, of the Prescription Monitoring Program Advisory Committee to serve as members of the peer review subcommittee. Effective immediately |
Placed on Calendar Order of 2nd Reading Feb 28th, 2018 Do Pass Public Health; 009-000-000
2/27/2018 |
|
|
SB3015 |
Koehler - Peoria, D |
Amends the School Code. With regard to the self-administration and self-carry of asthma medication, provides that a school district, public school, charter school, or nonpublic school may authorize a school nurse or trained personnel to (i) provide undesignated asthma medication to a student for self-administration only or to any personnel authorized under a student's Individual Health Care Action Plan or asthma action plan, plan pursuant to Section 504 of the federal Rehabilitation Act of 1973, or individualized education program plan to administer to the student that meets the student's prescription on file, (ii) administer an undesignated asthma medication that meets the prescription on file to any student who has an Individual Health Care Action Plan or asthma action plan, plan pursuant to Section 504 of the federal Rehabilitation Act of 1973, or individualized education program plan that authorizes the use of asthma medication; and (iii) administer an undesignated asthma medication to any person that the school nurse or trained personnel believes in good faith is having respiratory distress; defines "undesignated asthma medication" and "respiratory distress". Changes the definition of "asthma medication" to mean quick-relief asthma medication that is approved by the United States Food and Drug Administration for the treatment of respiratory distress. Provides that a school nurse or trained personnel may administer undesignated asthma medication to any person whom the school nurse or trained personnel in good faith believes to be experiencing respiratory distress (i) while in school, (ii) while at a school-sponsored activity, (iii) while under the supervision of school personnel, or (iv) before or after normal school activities. Provides that a school district, public school, charter school, or nonpublic school may maintain a supply of an asthma medication in any secure location where a person is most at risk. Provides that a training curriculum to recognize and respond to respiratory distress may be conducted online or in person. Specifies training requirements. Makes other changes. Effective immediately. Senate Amendment 1: Added must notify the child’s health care provider AND school nurse within 24 hours after the administration of an undesignated asthma medication. |
Sen. Amendments 1-3 adopted. Placed on 2nd Reading in the Senate
4/17/2018 |
|
|
SB3116 |
Hunter – Chicago, D |
Amends the Nurse Practice Act. In provisions concerning written collaborative agreements, restores the ability of podiatric physicians to collaborate with advanced practice registered nurses. Makes other changes. Effective immediately. |
Placed on 2nd Reading in the Senate
4/19/2018 |
|
|
SB3170 |
Stadelman - Rockford, D |
Amends the Pharmacy Practice Act and the Illinois Food, Drug and Cosmetic Act. Provides that a prescription for medication other than controlled substances shall be valid for up to 15 months from the date issued for the purpose of refills, unless the prescription states otherwise. |
On 3rd Reading in the Senate
4/18/2018 |
|
|
SB3431 |
Rezin - Peru, R |
Amends the Illinois Controlled Substances Act. Provides that when issuing a prescription for an opiate to a patient 18 years of age or older for outpatient use for the first time, a practitioner may not issue a prescription for more than a 7-day supply. Provides that a practitioner may not issue an opiate prescription to a person under 18 years of age for more than a 7-day supply at any time and shall discuss with the parent or guardian of the person under 18 years of age the risks associated with opiate use and the reasons why the prescription is necessary. Provides that notwithstanding this provision, if, in the professional medical judgment of a practitioner, more than a 7-day supply of an opiate is required to treat the patient's acute medical condition or is necessary for the treatment of chronic pain management, pain associated with a cancer diagnoses, or for palliative care, then the practitioner may issue a prescription for the quantity needed to treat that acute medical condition, chronic pain, pain associated with a cancer diagnosis, or pain experienced while the patient is in palliative care. Provides that the condition triggering the prescription of an opiate for more than a 7-day supply shall be documented in the patient's medical record and the practitioner shall indicate that a non-opiate alternative was not appropriate to address the medical condition. Provides that these provisions do not apply to medications designed for the treatment of substance abuse or opioid dependence. Effective immediately.
|
Referred to Assignments |
Neutral |
|
SB3498
|
Sims, Jr. -Chicago, D |
Amends the Managed Care Reform and Patient Rights Act. Requires a policy or plan sponsor to notify the prescribing physician and the patient in writing 60 days before making a formulary change that alters the terms of coverage or discontinues coverage for a prescribed drug that the patient is receiving. Contains provisions for receiving the notice electronically. Provides that a policy or plan sponsor may provide the patient with the written notification, along with a 60-day supply of the prescription drug, at the time the patient requests a refill. Provides that nothing in the provisions prohibits insurers or pharmacy benefit managers from using certain managed pharmacy care tools so long as an exception process is in place allowing the prescriber to petition for coverage a non-preferred drug if sufficient clinical reasons justify an exception to the normal protocol. |
Assigned to Insurance 2/27/2018 |
Neutral |
|
HB0274 |
Mussman – Schaumburg, D |
Amends the Pharmacy Practice Act. Provides that "practice of pharmacy" includes the prescribing and dispensing of hormonal contraceptive patches and self-administered oral hormonal contraceptives. Defines "hormonal contraceptive patch" as a transdermal patch applied to the skin of a patient, by the patient or by a practitioner, that releases a drug composed of a combination of hormones that is approved by the United States Food and Drug Administration to prevent pregnancy and "self-administered oral hormonal contraceptive" as a drug composed of a combination of hormones that is approved by the United States Food and Drug Administration to prevent pregnancy and that the patient to whom the drug is prescribed may take orally. Allows pharmacists to prescribe and dispense contraceptives to a person over 18 years of age and a person under 18 years of age only if the person has evidence of a previous prescription from a primary care or a women's health care practitioner. Requires the Department of Financial and Professional Regulation to adopt rules to establish standard procedures for pharmacists to prescribe contraceptives. Provides requirements for the rules to be adopted by the Department. Provides that all State and federal laws governing insurance coverage of contraceptive drugs and products shall apply to the provisions. |
2nd Reading in the House |
Support |
|
HB3479 SB1888 |
Feigenholtz – Chicago, D |
Amends the Medical Assistance Article of the Illinois Public Aid Code. In addition to other specified actions required under the Code, requires a managed care community network that contracts with the Department of Healthcare and Family Services to establish, maintain, and provide a fair and reasonable reimbursement rate to pharmacy providers for pharmaceutical services, prescription drugs and drug products, and pharmacy or pharmacist-provided services. Provides that the reimbursement methodology shall not be less than the current reimbursement rate utilized by the Department for prescription and pharmacy or pharmacist-provided services and shall not be below the actual acquisition cost of the pharmacy provider. Requires a managed care community network to ensure that the pharmacy formulary used by the managed care community network and its contract providers is no more restrictive than the Department's pharmaceutical program. Effective July 1, 2018. |
Referred to Rules Comm.
4/13/2018 |
|
|
HB4096 |
Harris – Chicago, D |
Amends the Medical Assistance Article of the Illinois Public Aid Code. Provides that the Department of Healthcare and Family Services shall require each Medicaid Managed Care Organization to list as preferred on the Medicaid Managed Care Organization's preferred drug list every pharmaceutical that is listed as preferred on the Department's preferred drug list. Provides that the Department shall not prohibit, or adopt any rules or policies that prohibit, a Medicaid Managed Care Organization from: (i) covering additional pharmaceuticals that are not listed on the Department's preferred drug list; or (ii) removing from the Medicaid Managed Care Organization's preferred drug list any prior approval requirements applicable under the Department's preferred drug list. Provides that the Department shall not require a Medicaid Managed Care Organization to utilize a single, statewide preferred drug list and shall not prohibit a plan from negotiating drug pricing concessions or rebates on any drug with pharmaceutical companies, unless otherwise required by federal law. Provides that no later than July 1, 2018, the Department shall develop a standardized format for all Medicaid Managed Care Organization preferred drug lists in cooperation with Medicaid Managed Care Organizations and stakeholders, including, but not limited to, community-based organizations, providers, and individuals or entities with expertise in drug formulary development. Requires each Medicaid Managed Care Organization to post its preferred drug list on its website without restricting access to enrolled members and to update the preferred drug list posted on its website within 2 business days of making any changes to the preferred drug list, including, but not limited to, any and all changes to requirements for prior approval. Effective immediately. |
Placed on Calendar Order of 3rd Reading - Short Debate
1/30/2018
|
|
|
HB4146 |
Fine – Glenview, D |
House Amendment 1 : n language providing that a health care plan is not prohibited from requiring a pharmacist to effect substitutions of prescription drugs, provides that the health care plan is not prohibited from requiring a pharmacist to effect substitutions consistent with provisions from the Pharmacy Practice Act that allow a pharmacist to substitute an interchangeable biologic for a prescribed biologic product and select a generic drug determined to be therapeutically equivalent by the United States Food and Drug Administration and in accordance with the Illinois Food, Drug and Cosmetic Act. |
Placed on 2nd Reading in the House
4/10/2018 |
|
|
HB4166 |
Harris – Chicago, D |
Creates the Health Insurance Claims Assessment Act. Imposes an assessment of 1% on claims paid by a health insurance carrier or third-party administrator. Provides that the moneys received and collected under the Act shall be deposited into the Healthcare Provider Relief Fund and used solely for the purpose of funding Medicaid services provided under the medical assistance programs administered by the Department of Healthcare and Family Services. |
Referred to Rules Comm.
4/13/2018 |
|
|
HB4270 |
Olsen - Downers Grove, R |
Amends the Good Samaritan Act. Provides that a free medical clinic shall not be liable for civil damages as a result of acts or omissions in providing medical treatment, diagnosis, or advice, except for willful or wanton misconduct. |
Referred to Rules Comm. 4/13/2018 |
|
|
HB4331 |
Conner – Romeoville, D |
Amends the Counties Code. Provides that in every case in which an opioid overdose is determined to be a contributing factor in a death, the coroner shall report the death and the age, gender, race, and county of residence, if known, of the decedent to the Department of Public Health. Amends the University of Illinois Hospital Act and the Hospital Licensing Act. Requires every hospital to report the age, gender, race, and county of residence, if known, of each patient diagnosed as having an opioid overdose to the Department within 48 hours of the diagnosis. Amends the Department of Public Health Powers and Duties Law of the Civil Administrative Code of Illinois. Requires the Department to adopt rules to implement the reporting requirements. Requires the Department to annually report to the General Assembly the data collected. |
Placed
on Calendar 2nd Reading- Short
3/8/2018 |
|
|
HB4436 |
Chapa LaVia -Aurora, D |
Amends the Mental Health and Developmental Disabilities Code. Provides that notwithstanding any of the provisions of the Code concerning the administration of psychotropic medication and electroconvulsive therapy, psychotropic medication or electroconvulsive therapy may be administered pursuant to a power of attorney for health care under the Powers of Attorney for Health Care Law or a declaration for mental health treatment under the Mental Health Treatment Preference Declaration Act over the objection of the recipient if the recipient has not revoked the power of attorney or declaration for mental health treatment as provided in the relevant statute. Effective immediately. |
Referred to Rules
1/31/2018
|
|
|
HB4440 |
Gabel - Evanston, D |
Amends the Nursing Home Care Act. Provides that the Department of Public Health shall provide facilities with educational information on all vaccines recommended by the Centers for Disease Control and Prevention's Advisory Committee on Immunization Practices, including, but not limited to, the risks associated with shingles and how to protect oneself against the varicella-zoster virus. Requires a facility to distribute the information to each resident who requests the information and each newly admitted resident. Allows the facility to distribute the information to residents electronically. Effective January 1, 2019. |
Arrived in Senate Referred to Assignments
3/9/2018 |
|
|
HB4479 |
Cabello – Rockford, R |
Amends the Criminal Code of 2012 relating to first degree murder. Adds and eliminates aggravating factors for which the death penalty may be imposed. Amends the Code of Criminal Procedure of 1963. Eliminates provision that abolishes the sentence of death. Enacts the Capital Crimes Litigation Act of 2018. Provides that all unobligated and unexpended moneys remaining in the Death Penalty Abolition Fund on the effective date of the amendatory Act shall be transferred into the Capital Litigation Trust Fund. Amends the State Appellate Defender Act. Provides that in cases in which a death sentence is an authorized disposition, the State Appellate Defender shall provide trial counsel with legal assistance and the assistance of expert witnesses, investigators, and mitigation specialists from funds appropriated to the State Appellate Defender specifically for that purpose by the General Assembly. Provides that the Office of State Appellate Defender shall not be appointed to serve as trial counsel in capital cases. |
Referred to Rules Committee
2/2/2018
|
|
|
HB4571
|
Ammons - Champaign, D |
Amends the Cannabis Control Act. Makes a technical change in a Section concerning the short title. |
Referred to Rules Committee
2/6/2018 |
|
|
HB4643
|
Burke - Chicago, D |
Amends the Illinois Physical Therapy Act. Provides that the limitation on determining a differential diagnosis shall not in any manner limit a physical therapist from establishing a relevant diagnosis. In the definition of "documented current and relevant diagnosis" and in provisions concerning disciplinary actions, removes language requiring a diagnosis to be substantiated by a physician, dentist, advanced practice registered nurse, physician assistant, or podiatric physician. Effective immediately. |
Placed on 2nd Reading in the House
4/10/2018 |
|
|
HB4650
|
Zalewski - Riverside, D |
Amends the Illinois Controlled Substance Act. In a provision allowing pharmacists to authorize a designee to consult the Prescription Monitoring Program on their behalf, defines "pharmacist" to include, but be not limited to, a pharmacist associated with a health maintenance organization or a Medicaid managed care entity providing services under the Illinois Public Aid Code. Effective immediately. |
On 2nd Reading in the House |
Oppose |
|
HB4696
|
Olsen - Downers Grove, R |
Amends the Use Tax Act, the Service Use Tax Act, the Service Occupation Tax Act, and the Retailers' Occupation Tax Act. Provides that, beginning on January 1, 2019, the tax is not imposed on prescription medicines or prescription drugs. Effective immediately. |
Referred to Rules Committee
2/13/2018 |
|
|
HB4699
|
Carroll - Buffalo Grove, D |
Amends the Good Samaritan Act. Provides that a person who in good faith and without fee administers epinephrine to another person who is suffering from a severe allergic reaction shall not be liable for civil damages as a result of his or her acts or omissions, except for willful or wanton misconduct on the part of the person in administering the epinephrine. |
Referred to Rules Committee
2/13/2018 |
|
|
HB4707 House Amendment 001 |
Scherer - Decatur, D |
House Amendment 003: Creates the Prescription Drug Task Force Act and a Prescription drug task Force with 18 members, one member from ICHP that will study the extent of over prescribing of opioids to patients and to make recommendations for future legislation to address the issue. |
Placed on Calendar 2nd Reading – Short Debate
3/8/2018 |
Neutral |
|
HB4795 |
Demmer – Rochelle, R |
Amends the Alcoholism and Other Drug Abuse and Dependency Act. Changes the short title of the Act to the Substance Use Disorder Act. Removes the terms "addict", "addiction", "alcoholic", "alcoholism", and "substance abuse" and their corresponding definitions. Requires the Department of Human Services to reduce the incidence of substance use disorders (rather than reduce the incidence and consequences of the abuse of alcohol and other drugs). Defines "substance use disorder". Requires the Department to design, coordinate, and fund prevention, early intervention, treatment, and other recovery support services for substance use disorders that are accessible and address the needs of at-risk individuals and their families. Requires the Department to develop a comprehensive plan on the provision of such services; assist other State agencies in developing and establishing substance use disorder services for the agencies' clients; adopt medical and clinical standards on how to determine a substance use disorder diagnosis; and other matters. Contains provisions concerning the licensing of substance use disorder treatment providers; licensure categories and services; the identification of individuals who need substance use disorder treatment using "SBIRT"; patients' rights; services for pregnant women, mothers, and criminal justice clients; and other matters. Repeals a provision of the Act establishing the Committee on Women's Alcohol and Substance Abuse Treatment. Repeals a provision of the Act setting forth the powers and duties of the Medical Advisory Committee. Makes conforming changes concerning the Substance Use Disorder Act to several Acts including the Department of Human Services Act, the Children and Family Services Act, and the Mental Health and Developmental Disabilities Administrative Act. Effective January 1, 2019. |
Placed on 2nd Reading in the House
4/10/2018 |
|
|
HB4825 |
Evans, Jr – Chicago, D |
Amends the Illinois Insurance Code. Provides that all entities providing prescription drug coverage shall permit and apply a prorated daily cost-sharing rate to prescriptions that are dispensed by a pharmacy for less than a 30-day supply if the prescriber or pharmacist indicates the fill or refill could be in the best interest of the patient or is for the purpose of synchronizing the patient's chronic medications. Provides that no entity providing prescription drug coverage shall deny coverage for the dispensing of any drug prescribed for the treatment of a chronic illness that is made in accordance with a plan among the insured, the prescriber, and a pharmacist to synchronize the refilling of multiple prescriptions for the insured. Provides that no entity providing prescription drug coverage shall use payment structures incorporating prorated dispensing fees determined by calculation of the days' supply of medication dispensed. Provides that dispensing fees shall be determined exclusively on the total number of prescriptions dispensed. Establishes criteria for an entity conducting audits (either on-site or remotely) of pharmacy records. Provides that the Department of Insurance and Director of Insurance shall have the authority to enforce the provisions of the Act and impose financial penalties. Effective January 1, 2019. |
Rules Committee
2/14/2018 |
|
|
HB4900 |
Guzzardi - Chicago, D |
Creates the Illinois Generic Drug Pricing Fairness Act. Provides that a manufacturer or wholesale drug distributor shall not engage in price gouging in the sale of an essential off-patent or generic drug. Provides that the Director of Healthcare and Family Services or Director of Central Management Services may notify the Attorney General of any increase in the price of any essential off-patent or generic drug under the Medical Assistance Program under the Illinois Public Aid Code or a State health plan, respectively, that amounts to price gouging. Provides that whenever the Attorney General has reason to believe that a manufacturer or wholesale drug distributor of an essential off-patent or generic drug has violated the Act, the Attorney General shall send a notice to the manufacturer or wholesale drug distributor requesting a specified statement. Provides that within 45 days after receipt of the request, the manufacturer or wholesale drug distributor shall submit the statement to the Attorney General. Provides that to accomplish the objectives and carry out the duties prescribed in the Act, the Attorney General may issue subpoenas or examine under oath any person to determine whether a manufacturer or wholesale drug distributor has violated the Act. Provides that upon petition of the Attorney General, a circuit court may issue specified orders against violations of the Act. Contains provisions concerning the disclosure of financial information provided by a manufacturer or wholesale drug distributor to the Attorney General. Effective January 1, 2019. |
Held on 3rd Reading in the House
4/19/2018 |
|
|
HB4907 SB2952 |
McAuliffe - Chicago, R |
Amends the Illinois Controlled Substances Act. Provides that the Department of Human Services, in consultation with the Advisory Committee, shall adopt rules allowing licensed prescribers or pharmacists who have registered to access the Prescription Monitoring Program to authorize a licensed or non-licensed designee (rather than any designee) employed in that licensed prescriber's office or licensed pharmacist's pharmacy and who has received training in the federal Health Insurance Portability and Accountability Act to consult the Prescription Monitoring Program on their behalf. House Amendment 1 now requires the designee in the pharmacy must be a licensed individual. Requires the Clinical Director of the Prescription Monitoring Program to select 6 members (rather than 5 members), 3 physicians, 2 pharmacists, and one dentist, of the Prescription Monitoring Program Advisory Committee to serve as members of the peer review subcommittee. Effective immediately |
Placed on 2nd Reading in the House
4/10/2018
|
|
|
HB4950 SB2951 |
Feigenholtz – Chicago, D |
Creates the Early Mental Health and Addictions Treatment Act. Requires the Department of Healthcare and Family Services, and other specified agencies and entities, to develop a pilot program under which a qualifying adolescent or young adult may receive community-based mental health treatment from a youth-focused community support team for early treatment that is specifically tailored to the needs of youth and young adults in the early stages of a serious emotional disturbance or serious mental illness. Requires the Department to apply, no later than September 30, 2019, for any necessary federal waiver or State Plan amendment to implement the pilot program. Requires the Department to implement the pilot program no later than December 31, 2019 if federal approval is not necessary. Contains provisions concerning the creation of a community-based treatment model under the pilot program; the development of a pay-for-performance payment model; Department rules to implement the pilot program; and analytics and outcomes report. Requires the Department to develop an Assertive Engagement and Community-Based Clinical Treatment Pilot Program for individuals with opioid and other drug addictions. Contains provisions on in-office, in-home, and in-community services provided under the pilot program; application for a federal waiver or State Plan amendment to implement the pilot program; development of a pay-for-performance payment model; Department rules to implement the pilot program; and analytics and outcomes report. Effective immediately. |
Referred to Rules Committee
2/14/2018
Added Chief Co-Sponsor
3/12/2018 |
|
|
HB4995 |
Crespo-Streamwood, D |
Amends the Illinois Insurance Code and the Illinois Public Aid Code. Requires that on or before July 1, 2019, the Department of Insurance and Department of Healthcare and Family Services to jointly develop a uniform prior authorization form to be used by prescribing providers to request prior authorization for prescription drug benefits. Provides that on and after January 1, 2020, or 6 months after the uniform prior authorization form is developed, whichever is later, health insurers, managed care organizations, and fee-for-service medical assistance programs that provide prescription drug benefits shall utilize and accept the uniform prior authorization form and prescribing providers may use the uniform prior authorization form. Provides criteria for developing the uniform prior authorization form. Provides requirements and limitations of prior authorization requests. |
Rules Comm. |
|
|
HB5070 |
Bellock – Westmont, R |
Amends the Telehealth Act. Includes clinicians licensed to provide medical services under Illinois law in the definition of "health care professional". Amendment 001: Added pharmacists and other licensed practitioners under the definition of “health care professional”. |
Passed in the House 4/18/2018
Referred to Assignments Comm. in the Senate. |
|
|
HB5442 |
Durkin - Burr Ridge, R |
Amends the Open Meetings Act. Provides that, for the purposes of the Act, "public body" does not include a Metropolitan Enforcement Group (MEG) Policy Board or drug task force composed or created by any combination of local law enforcement agencies. Amends the Criminal Code of 2012. Provides that a person commits drug-induced homicide when he or she violates delivery of a controlled substance or methamphetamine or a similar law of another jurisdiction, by unlawfully delivering a controlled substance to another, and the injection, inhalation, absorption, or ingestion of any amount of that controlled substance is a contributing cause of the person's death. Amends the Illinois Controlled Substances Act. Provides that controlled substances which are lawfully administered in hospitals or institutions licensed under the Hospital Licensing Act shall be reported under (rather than, exempt from) specified reporting provisions under the Act, and the prescription for the controlled substances ordered and the quantity actually administered (rather than, the reporting requirement only applies for more than a 72-hour supply of a discharge medication to be consumed outside of the hospital or institution). Provides that the information required to be transmitted under the prescription monitoring program must be transmitted not later than the end of the business day on which a controlled substance is dispensed, or at such other time as may be required by the Department of Human Services by administrative rule (rather than, at the end of the next business day on which the controlled substance is dispensed). |
Tabled 04/12/2018
|
Neutral |
|
HB5482 |
Guzzardi – Chicago, D |
Amends the Department of Professional Regulation Law of the Civil Administrative Code of Illinois. Provides that the Department of Financial and Professional Regulation shall allow an applicant to provide his or her individual taxpayer identification number as an alternative to provide a social security number when applying for a license. Provides that no applicant shall be denied a license solely based on his or her immigration status or citizenship status. Makes conforming changes in the School Code, Pharmacy Practice Act, and the Attorney Act. Makes other changes. |
Placed on 2nd Reading in the House
4/10/2018 |
|
|
HB5602 |
Carroll - Buffalo Grove, D |
Amends the Safe Pharmaceutical Disposal Act. Provides that "unused medication" means any unopened, expired, or excess medication that has been dispensed for patient or resident care and that is in a liquid or solid form (rather than in a solid form). Makes related changes. |
Referred to Rules Committee 2/16/2018
Added Co-Sponsor 3/12/2018 |
|
|
HB5747 |
Mussman - Schaumburg, D |
Amends the Pharmacy Practice Act. Provides that "practice of pharmacy" includes the prescribing and dispensing of hormonal contraceptive patches and self-administered oral hormonal contraceptives. Defines "hormonal contraceptive patch" as a transdermal patch applied to the skin of a patient, by the patient or by a practitioner, that releases a drug composed of a combination of hormones that is approved by the United States Food and Drug Administration to prevent pregnancy and "self-administered oral hormonal contraceptive" as a drug composed of a combination of hormones that is approved by the United States Food and Drug Administration to prevent pregnancy and that the patient to whom the drug is prescribed may take orally. Allows pharmacists to prescribe and dispense contraceptives to a person over 18 years of age and a person under 18 years of age only if the person has evidence of a previous prescription from a primary care or a women's health care practitioner. Requires the Department of Financial and Professional Regulation to adopt rules to establish standard procedures for pharmacists to prescribe contraceptives. Provides requirements for the rules to be adopted by the Department. Provides that all State and federal laws governing insurance coverage of contraceptive drugs and products shall apply to the provisions. |
Referred to Rules Committee 2/16/2018
Added Co-Sponsor Reps 3/8/2018 |
Support |
Senate Deadlines House Deadlines
Bill Introduction February 16, 2018 February 16, 2018 Bill Introduction
Senate bills out of Committee April 13, 2018 April 13, 2018 House Bills out of Committee
Third Reading for Senate Bills April 27, 2018 April 27, 2018 Third Reading House Bills
House Bills out of Committee May 11, 2018 May 18, 2018 Senate Bills out of Committee
Third Reading House Bills May 25, 2018 May 25, 2018 Third Reading Senate Bills
Adjournment May 31, 2018 May 31, 2018 Adjournment
New Practitioner Network
2017 Leadership Retreat in the Eyes of a Student and Resident
by Hyerim Whang Kong Doctor of Pharmacy Candidate 2019 University of Illinois at Chicago; Bridget Dolan, PharmD PGY-2 Ambulatory Care Pharmacy Resident Northwestern Memorial Hospital
Student Perspective:
The 2017 ICHP Leadership Retreat was held at the Allerton Park and Retreat Center in Monticello, Illinois. The retreat began around noon on Friday with lunch and an ice breaker session. The group started with the leadership session led by ICHP President, Travis Hunerdosse, PharmD, MBA. His presentation focused on how to strengthen leadership skills. This session was informative, interactive, and was truly enjoyed. All participants shared their knowledge and experiences that they went through as a leader. After this session, the group had free time to enjoy the weather and the hiking that Allerton offers. After the hike, we attended dinner and the social networking hour. During that time, I could meet and talk to individuals and hear personal stories, both pharmacy and non-pharmacy related.
On Saturday, the group started early in the morning with a session led by each Division Director. The goal of this session was to celebrate successes within each Division and identify topics that will help ensure the continued success of ICHP and advocacy of pharmacy. It was helpful for me as a student to realize how important it is to advocate for the pharmacy profession. Often, students do not fully understand why advocating for their profession is important. However, this retreat showed just how important it is. Personally, I was unaware of the multi-level impact and how important it is to keep up to date on pharmacy related laws. After this leadership retreat, I learned that a small amount of time can make a big difference, particularly when large numbers of people act together towards a common goal. As a student leader of ICHP, it is my job to advertise why advocacy of the profession is important to pharmacy students and pharmacists.
As a student, I was hesitant to attend the leadership retreat at first because I felt overwhelmed by the unfamiliarity of the event. However, when I arrived everyone welcomed me and treated me as if I had attended before. They shared their experiences as clinical pharmacists and as ICHP leaders, which made me challenge myself to become a better student and a better leader of the ICHP student chapter. I would like to thank the ICHP leaders who gave me opportunity to attend the 2017 Leadership Retreat. When comparing the past to the present, it is clear that the duty of pharmacists in modern society is changing at a fast pace. When pharmacy students are involved in the issues affecting pharmacy, and we take steps to influence policy through the legislative and regulatory process, we can help shape the profession that we will be entering in the future.
Resident Perspective:
In October 2017, I had the opportunity to attend the ICHP Leadership Retreat at the Allerton Park & Retreat Center in Monticello, Illinois. Normally, this retreat is only for pharmacist and pharmacy technician leaders in ICHP, but I was lucky to go as a member with my rotation preceptor at the time and ICHP President, Travis Hunerdosse, PharmD, MBA. I did not know what to expect from this retreat, but I was pleasantly surprised at all the information I learned and how fueled with energy I became during the discussions about the pharmacy profession. During the group discussion, many items were brought up about advancing the pharmacy profession through the pharmacist and pharmacy technician roles. Listening to the ideas of the ICHP leaders and bouncing around my ideas as a new practitioner helped to keep the conversation going. The biggest thing I took away from the weekend experience was how important it is to get involved in an organization. This involvement might be monthly committee calls, attending leadership events, or volunteering for events. The more people you can connect with, the better it is for the profession. I met so many practitioners from all over the state, and it is always helpful to form a network of pharmacists and pharmacy technicians to help progress the role of pharmacy in healthcare. Pharmacy technician development is just as important as the pharmacist because we depend on each other and need to work together at the top of our licenses. This retreat allowed pharmacy technicians to get involved in leadership roles and discuss how to increase technician involvement in organizations. Since the retreat, I have become an active member of the New Practitioners Network and the Ambulatory Care Network to keep networking and learn how others are practicing throughout the state of Illinois.
Professional Affairs - Call for Entries
Best Practice Award!

Past winners include:
2017
Sandra M. Salverson, Pharm.D., BCPS and Jerry Storm, RPh
Implementation of Integrated Telepharmacy Services Achieve a Health-System Standard of Pharmacy Care
2016
Maya Beganovic, PharmD and Sarah M. Wieczorkiewicz, PharmD, BCPS
"MALDI-TOF alone versus MALDI-TOF combined with real-time antimicrobial stewardship interventions on time to optimal therapy in patients with positive blood cultures"
2015
Kuntal Patel, Pharm.D., Pavel Prusakov, and Heather Vaule
"Osteopenia of Prematurity (aka Better Bones for Babies)"
2014
Arti Phatak, Pharm.D.; Brooke Ward, Pharm.D., BCPS; Rachael Prusi, Pharm.D.; Elizabeth Vetter, Pharm.D.; Michael Postelnick, BS Pharm, BCPS (AQ Infectious Diseases); and Noelle Chapman, Pharm.D., BCPS
"Impact of Pharmacist Involvement in the Transitional Care of High-Risk Patients through Medication Reconciliation, Medication Education, and Post-Discharge Callbacks"
View 2008 - 2013 Winners at ichpnet.org
Online entry form: http://www.ichpnet.org/professional_practice/best_practices/
Submission deadline: July 1, 2018
The objective of the Best Practice Award program is to encourage the development of innovative or creative pharmacy practice programs or innovative approaches to existing pharmacy practice challenges in health systems within the state of Illinois.
Applicants will be judged on their descriptions of programs and practices employed in their health system based on the following criteria:
- Innovativeness / originality
- Contribution to improving patient care
- Contribution to institution and pharmacy practice
- Scope of project
- Quality of submission
Eligibility
Applicants must be a member of ICHP for a minimum of 90 days prior to the submission deadline and practice in a health system setting. More than one program can be submitted by a health system for consideration.
Instructions for preparing manuscript
Each entry for the Best Practice Award must include a manuscript prepared as a Word document, double-spaced using Times New Roman 12-pitch type. A header with the paper title and page number should appear on each page. The manuscript should not exceed 2000 words in length (not counting references), plus no more than a total of 6 supplemental graphics (tables, graphs, pictures, etc.) that are relevant to the program. Each picture, graph, figure, and table should be mentioned in the text and prepared as a separate document clearly labeled.
The manuscript should be organized as a descriptive report using the following headings:
- Introduction, Purpose, and Goals of the program
- Description of the program
- Experience with and outcomes of the program
- Discussion of innovative aspects of programs and achievement of goals
- Conclusion
Format
Submissions will only be accepted via online submission form. The manuscript will be forwarded to a pre-defined set of reviewers. Please do not include the names of the authors or affiliations in the manuscript to preserve anonymity.
All applicants will be notified of their status within three weeks of the submission deadline. Should your program be chosen as the winner:
- The program will be featured at the ICHP Annual Meeting. You will need to prepare a poster to present your program and/or give a verbal presentation. Guidelines will be sent to the winner.
- You will be asked to electronically submit your manuscript to the ICHP KeePosted for publishing. This program will be accredited for CPE and will require that you complete material for ACPE accreditation.
- You will receive a complimentary registration to the ICHP Annual Meeting, recognition at the meeting and a monetary award of $1,000 distributed to your institution.
Non-winning submissions may also be considered for publication in the ICHP KeePosted, but your permission will be obtained beforehand.
If you have any questions related to the program please contact Trish Wegner at trishw@ichpnet.org.
Thank you to PharMEDium, Division of AmerisourceBergen for providing a grant for this year's Best Practice Award!
The 2016 & 2017 Best Practice Winners Available for CPE!
MALDI-TOF alone versus MALDI-TOF combined with real-time antimicrobial stewardship interventions on time to optimal therapy in patients with positive blood cultures
Authors: Maya Beganovic, PharmD and Sarah M. Wieczorkiewicz, PharmD, BCPS
Advocate Lutheran General Hospital, Park Ridge, Illinois
Implementation of Integrated Telepharmacy Services Achieve a Health-System Standard of Pharmacy Care
Authors: Sandra M. Salverson, PharmD, BCPS and Jerry Storm, RPh
OSF Healthcare System, Peoria, Illinois
Click here to learn how ICHP members can obtain free CPE credit.
Features
Nominate a Winner!
ICHP Awards Process Opens
Feature Article
by Scott A. Meyers, Executive Vice President
It’s that time of year. ICHP is looking for Illinois Pharmacy’s best and brightest! The nominations process for the 2018 ICHP Pharmacist of the Year, the Amy Lodolce Mentorship Award, and the Pharmacy Technician of the Year recipients is open, and it’s your chance to recommend someone you know and respect. All three awards will be presented at the 2018 ICHP Annual Meeting on September 13-15, 2018 at the Drury Lane Theater in Oakbrook Terrace along with several other important awards. This is your chance to help select some of Illinois pharmacy’s finest!
The process is different for each award, so let’s start with ICHP’s highest honor, the Pharmacist of the Year.
Pharmacist of the Year Award
A Pharmacist of the Year nominee should meet the following criteria:
· The nominee is a person of high moral character, good citizenship and high professional ideals;
· The nominee has made significant contributions affecting the practice of health-system pharmacy throughout the state; and
· These contributions should be in the form of sustained exemplary service in health-system pharmacy or a single outstanding achievement, or a combination of accomplishments benefiting health-system pharmacy, and through it, humanity and the public health. These accomplishments, achievements, or outstanding performances may be in the following areas:
o Health-system pharmacy practice
o Health-system pharmacy education
o Health-system pharmacy administration
o Pharmaceutical research or development related to health-system pharmacy
o Pharmacy organizational activity with a definite relationship to health-system pharmacy
o Scientific or professional pharmacy writing, (e.g., noteworthy articles on pharmaceutical subjects with applicability to health-system pharmacy)
o Pharmaceutical journalism related to health-system pharmacy
o Public and/or inter-professional relations activities benefiting health-system pharmacy
o Pharmacy law or legislation, professional regulations, standards of professional conduct, or pharmacy law enforcement as related to health-system pharmacy.
Nominations may be received from Selection Committee members (past recipients of the award), past Presidents of the Council, affiliated chapters of the Council or any six pharmacist members of the Council submitting and signing a recommendation. Nominators should write a complete nomination letter and submit it along with the nominee’s CV by July 2nd, to the ICHP office at scottm@ichpnet.org . Nominations should include the name of the nominee and details describing how they meet the above criteria. This year’s Selection Committee Chair is last year’s recipient, Desi Kotis. All nominations will be forwarded to the ICHP office for distribution to the selection committee.
Amy Lodolce Mentorship Award
Amy Lodolce was a University of Illinois at Chicago College of Pharmacy faculty member who touched the lives of pharmacy students, residents, and colleagues through her passion for teaching and the profession of pharmacy. Throughout her time at the college, Amy oversaw the training of four PGY2 drug information pharmacy residents, all of whom are currently drug information faculty at various institutions. She worked directly with numerous PGY1 residents and APPE students during their drug information rotations. She also served as a formal mentor to her student advisees and was the advisor of the Phi Delta Chi pharmacy fraternity for many years. As the Assistant Director of the Drug Information Group, Amy served as an informal mentor to other faculty and was quick to help new faculty become oriented and situated.
Amy approached being a leader and a mentor with an “open door” policy, and she would selflessly pause her work to address others’ needs. Students, residents, and faculty alike would ask her for guidance with career decisions and other professional concerns. Amy was respectful and nonjudgmental in her approach when assisting others whose goals and aspirations may have been different from her own. Her dedication was exemplary in that she worked tirelessly to provide residents and students with quality learning opportunities. She led and coached by example, consciously choosing behaviors that she hoped students and residents would emulate. An active pharmacist member of ICHP, Amy placed emphasis on professional organization involvement and giving back to the profession. Amy’s dedication and generosity to the profession of pharmacy have positively shaped many pharmacists’ careers, and the memory of her will continue to do so.
Award Criteria:
· The individual nominated to receive this award must be an ICHP pharmacist, associate or technician member in good standing;
· The individual should be an exemplary preceptor, professor and/or mentor of students, residents, pharmacy technicians and/or new practitioners;
· The individual should be a positive role model for pharmacists, pharmacy students and/or pharmacy technicians;
· In order to be considered for the award, individuals must have been nominated using the approved nomination form below;
· More than one person may complete a nomination form for an individual.
To nominate someone for the Amy Lodolce Mentorship Award:
1. Please provide your name(s), i.e., the name of the nominator(s). (More than one person can nominate a nominee).
2. Provide the name of the person you are nominating. In addition, the nominee’s curriculum vitae must be included in the nomination package.
3. Please answer the following questions about the nominee:
a. Is the nominee a member of ICHP?
b. In what capacity have you worked with the nominee?
c. In what ways do you see the nominee working to advance the profession of pharmacy?
d. Give some examples of ways in which this nominee is a model mentor/preceptor.
e. Give some examples in which this nominee has demonstrated a service to community (outside of job responsibilities).
f. How has this nominee impacted your career?
Completed nominations should be sent by July 2, 2018, to Scott Meyers at scottm@ichpnet.org or to the ICHP office by fax at 815-227-9294 or mail to 4055 N. Perryville Rd., Loves Park, IL 61111.
ICHP Technician of the Year Award
Award Purpose: The ICHP Pharmacy Technician of the Year Award is established to identify and recognize exceptional performance by a certified pharmacy technician within the State of Illinois.
Award Criteria:
The candidate must:
· Be a current ICHP technician member,
· Be a PTCB certified pharmacy technician for at least two years,
· Demonstrate at least one of the following:
- exceptional contributions to his/her pharmacy worksite
- exceptional contributions to ICHP as a volunteer member
- exceptional contributions to the practice of pharmacy in Illinois
The nominator must:
· Be the technician’s supervisor, colleague or co-worker. No self-nominations will be accepted.
· Provide the following information:
o Worksite name, address and telephone number.
o Technician name and year certified.
o Describe the technician’s contributions in detail.
o Provide the nomination to the ICHP office (scottm@ichpnet.org) by no later than July 2nd.
Selection Process:
· Deadline for nominations is July 2, 2018.
· All nominations are reviewed by the Division of Marketing Affairs at their July conference call.
· The Division will select two finalists for consideration and present them to the ICHP Board of Directors at the July Board Meeting.
· The ICHP Board of Directors will select the award winner from the two finalists presented by the Division of Marketing Affairs.
· The Division of Marketing Affairs may recommend one of the two finalists by providing detailed discussion points to the Board of Directors.
· The Board is not required to select a recipient if no candidate seems qualified.
Award:
· The Award recipient and her/his nominator will be notified immediately following his/her selection by the Board of Directors.
· The award recipient will receive a complimentary full registration to the ICHP Annual Meeting.
· The award recipient will receive a plaque to be presented at an appropriately agreed upon time during the ICHP Annual Meeting.
Again, the deadline for submissions of all award nominees is July 2, 2018 and they should be forwarded to the ICHP office.
College Connection
Chicago State University - College of Pharmacy
Nontraditional IPPE in Public Health: A Truly Invaluable Journey
College Connection
by Sara Shakhwar, SSHP P-1 Class Liaison
As my first year at Chicago State University-- College of Pharmacy comes to a close, I have been reflecting on my educational experiences both on and off campus. Having completed two introductory pharmacy practice experiences (IPPEs), I have learned a great deal about the profession and the world of healthcare. For my public health practicum, I was assigned to Northwest Special Recreation Association (NWSRA) Pursuit and Star Academy programs. This rotation was held during spring break – 45 hours of hands on experience in Pursuit, an adult day program for adults with special needs and Star Academy, an after-school program for children and teens with special needs.
Of course, my expectations as a student pharmacist were to be exposed to medicine, research, and clinical/retail settings during my IPPEs and eventually during my advanced pharmacy practice experiences. Before beginning with NWSRA, I was slightly taken off guard by the fact that I was not to be placed in a setting where I would be wearing my white coat and dealing with patients in the traditional sense. I soon discovered that each participant in the program is cared for exceptionally by the wonderful and dedicated staff. However, my specific role as a student pharmacist at the site was not initially clear to me. Slowly but surely, I began to discover my place.
As the week progressed, I was better able to interact and to communicate with the participants. For example, one individual responded well only when direct eye contact was made while another only liked certain snacks. We were able to develop a sense of what worked best for each participant and accommodate to their individual needs. We were also able to delve into their medical history and learn more about their specific disability, how it presented, and what medications were used to manage which conditions. We were able to observe how the medications were benefitting them, as well as how they experienced some apparent adverse effects, such as irritability or drowsiness.
While I had started the week at NWSRA with reservations about how the site would benefit me as a pharmacist, I ended the week with more knowledge and eye-opening experiences than I had imagined. I am a firm believer that education through practice is extremely important. This IPPE allowed me, as a future health care professional, to learn more about empathy and deal with people of all needs. I feel better prepared to interact with any patient that I encounter and now feel at ease in working with patients that have special needs. Our goal as pharmacists is to provide exceptional patient care; my experience during my public health rotation brought me one step closer to this goal.
Southern Illinois University Edwardsville (SIUE) - School of Pharmacy
Sold! …to the Highest Bidder: The SIUE SSHP Faculty Auction
College Connection
by James Reimer, P2, SIUE SSHP President-Elect & Paris Smith, P2, SIUE SSHP Fundraising Chair
Two words that do not seem to go well together are “Faculty” and “Auction”, yet they continue to pave the way for numerous rewarding experiences for our students. Now it might seem at first glance that the students of SIUE are trying to sell their professors, but rest assured that is not the case. The Faculty Auction fundraiser, sponsored by the SIUE Student Society of Health-System Pharmacists (SSHP), is a big hit with both the students and faculty. Not only does the Faculty Auction raise money for the student chapter of ICHP, it also promotes a unique opportunity for students to interact more closely with their faculty outside of the classroom setting. On top of that, the SIUE SSHP donates 10% of the profits from the auction to Ronald McDonald House Charities of Metro Saint Louis. We are proud to announce that we raised a record-breaking amount this year!
Every spring semester, in place of a regular scheduled general meeting, our SSHP hosts a special meeting called the Faculty Auction. At this meeting, our SSHP officers bring in food to be served as a potluck during the event. This event consists of SIUE faculty donating their time and money on a wide range of experiences on which the students can bid. The auction this year included an all-day BBQ tour around St. Louis, an all-day boating adventure on the lake, and multiple opportunities to have lunch or supper with the faculty throughout the local area. Other opportunities to win a casual interaction with the faculty included a night of bowling, an NCAA Final Four watch party, a Springfield faculty backyard picnic, multiple escape room challenges, a night of theatre to watch the award-winning musical, The Book of Mormon, and a dessert of the month experience. New this year was the opportunity for faculty to submit baskets in addition or in place of an experience with them.
This event takes quite an enormous amount of planning to pull off smoothly and successfully. The task is assigned to and led by our SSHP Fundraising Chair, but is carried out through teamwork. In this regard, our executive board members of SSHP divide and conquer by splitting up the faculty members. Each board member approaches their assigned faculty member(s) to ask them if they would like to donate an experience or a basket to the Faculty Auction. After faculty members agree to participate, we fill out forms for them that include the specific details and requirements of their auction item(s). The information from these forms are compiled into a PowerPoint document to include enticing graphics and descriptions to be displayed during the meeting. Auction items can be donated from an individual faculty member, a few faculty members, or even a large group of faculty members such as those from the Springfield area hosting a backyard picnic. Additionally, prizes can be for individual students, a few students, or a large group of students such as in all-day boating adventure. One of the truly fun aspects of the Faculty Auction is that each basket or event can be anything the faculty member thinks up. These auction items can be from a personal interest such as taking students to a concert to a hobby such as providing home cooked BBQ or desserts.
Once the auction has ended, we send out emails to the students and faculty of the winning bids. This allows the students and faculty to work out when their event will take place or when they can pick up their basket. This allows our students and faculty to get to know one another better. Students and faculty take advantage of this opportunity to discuss the curriculum, share their thoughts about our pharmacy program, and to simply network and establish relationships with each other. The Faculty Auction is an extremely popular event that not only serves to function as a means to raise funds for our organization and local charity, but also to facilitate meaningful out-of-classroom adventures and experiences between students and faculty. It is our hope here at SIUE, by sharing our experiences with other schools of pharmacy that they might adopt this fundraiser and see how much fun hanging out with your faculty can be!
“As a P1 member of SSHP last year, the Faculty Auction was one of my favorite events throughout the year and it allowed me to go to lunch with a professor and try a new cuisine. It also allowed me to ask her questions and give me advice about how to navigate through pharmacy school. It was an experience I will never forget, and I am proud to have headed the auction this year as fundraising chair and to have a record-breaking year. It allows us as students to get to know our faculty as our future colleagues and to enjoy spending time with them. I can’t wait to see what items we will get next year!” –Paris Smith, P2, SSHP Fundraising Chair
University of Illinois at Chicago-Rockford
Developing Clinical Skills, Pharmacy Knowledge, and Teamwork – Local Clinical Skills Competition at UIC- COP
College Connection
by Mehul Amin (P3), Professional Practice Chair, UIC-ICHP Chapter
Every year our ICHP student chapter from the University of Illinois at Chicago-Rockford hosts the local Clinical Skills Competition (CSC) for both campuses. The competition is open to all students, from P1s to P4s. The CSC is a highly interactive case-based scenario followed by an oral presentation to a panel of clinical faculty judges. The local CSC is a great opportunity for students to practice what they have learned in their therapeutics curriculum, and further develop their pharmacy knowledge, teamwork, and clinical skills.
This year we had a total of 13 (2-person) teams compete in the local competition held on campus this September and October 2017. Among the 13 teams (from both campuses), only one team is chosen to advance and represent our University at the national level, which was held at the ASHP Midyear Clinical Meeting in December 2017. This year Dovydas Krolis (P4) and Francisco Castaneda (P4) from the Rockford Campus blew the judges away, and got the opportunity to represent UIC COP at the ASHP Midyear Clinical Meeting which was held in Orlando, Florida.
They faced over a hundred teams from the best schools in the nation. The competition at the national level was similar to the local, however, the cases were far more complex and multifaceted. They had the opportunity to put their pharmacy skills to the test and apply everything they have learned at the competition.
Overall the national CSC is a wonderful experience, and despite not qualifying for Round 2 of the competition, the two P4s were not discouraged. They stated that it was highly rewarding for them to be able to utilize all the hard work they put into learning and studying clinical practice at the competition. Local and national level variations previously discussed aside, the competition overall was a wonderful opportunity for them to make connections with other like-minded peers from across the United States and represent the college at the national level.

Pictured: Dovydas Krolis (left) &
Francisco Castaneda (right)
UIC College of Pharmacy UIC-Rockford
More
Welcome New Members!
|
New Member |
Recuriter |
|
Mozhan Afshari |
|
|
Lindsey Berg |
|
|
Brittany Brozek |
|
|
Hurdaya Burns |
|
|
Beth Cady |
Carrie Vogler |
|
Monica Choi |
|
|
Suhi Choi |
Paige Taylor |
|
Courtney Darrah |
Ginger Ertel |
|
Rachel Didion |
|
|
Dawid Dymon |
|
|
Fjoralba Hoxha |
|
|
Thai Huynh |
|
|
Anna Jackson |
|
|
Kofi Jantuah |
|
|
Jenna Kalsow |
|
|
Sushil Khan |
|
|
Cindy Li |
|
|
Christine Mirretti |
|
|
Chelsea Obaroghedo |
|
|
Juvaria Rahman |
|
|
Andre Roberts |
|
|
Nicholas Sakinsky |
Tina Kakish |
|
Latrice Sparks |
|
|
Alexandra Steevensz |
|
|
Zlatiyan Stoykov |
|
|
Osama Umar |
|
|
Emmanuel Yemoh |
|
Upcoming Events

Pharmacy Directors Network – Chicago Area
Tuesday, May 15th, 2018 – 6:00pm
Francesca's on Taylor
1400 W Taylor St
Chicago, IL 60607
(312) 829-2828
Schedule:
5:30 pm Social Hour
6:00 pm Dinner Ordered
8:30 pm Depart
Dinner cost will be $45. Your credit card will be billed following the event but failure to attend with a confirmed reservation will incur the charge.
The Pharmacy Director Network meets bi-monthly and is open to ICHP member directors or their administrative designees.
This program is not open to students, technicians or pharmacy staff.
For more information about the network, please contact Scott Meyers at scottm@ichpnet.org.
West Central LIVE Presentation
Methicillin-Susceptible Staphylococcus Aureus
Bacteremia:
Clinical Pearls and Treatment Principles
Tuesday, May 22, 2018 - 6:00 pm
OSF St. Francis Medical Center - 7th Floor Board Room, Peoria, IL
Ashley Walter, PharmD
Save the date! Watch the CPE Newsbrief for information.
Topic: Safe Management of Drug Shortages
Thursday, July 12, 2018 - 12:00 noon
Speaker: Natasha Nicol, PharmD, FASHP

Officers and Board of Directors
Executive Officers
 Travis Hunerdosse
Travis Hunerdosse
President
(312) 926-6124
thunerdo@nm.org
 Charlene Hope
Charlene Hope
Immediate Past President
(708) 783-5933
chope@macneal.com
 Noelle Chapman
Noelle Chapman
President-Elect
 Kathryn Schultz
Kathryn Schultz
Treasurer
(312) 926-6961
kathryn_schultz@rush.edu
Regional Directors
 Amy Boblitt
Amy Boblitt
Regional Director Central
(217) 788-3015
boblitt.amy@mhsil.com
 Elise Wozniak
Elise Wozniak
Regional Director Northern
elise.m.wozniak@gmail.com
 Lynn Fromm
Lynn Fromm
Regional Director
Southern
(618) 391-5539
fromml@andersonhospital.org
Division Directors
 Mary Lee
Mary Lee
Organizational Affairs Director
(630) 515-7311
mleexx@midwestern.edu
 Karin Terry
Karin Terry
Professional Affairs Director
(309) 655-3390
Karin.l.terry@osfhealthcare.org
 Lara Ellinger
Lara Ellinger
Educational Affairs Director
(312) 926-3571
laelling@nm.org
 Carrie Vogler
Carrie Vogler
Marketing Affairs Director
(217) 545-5394
cvogler@siue.edu
Technician Representative
Network and Committee Chairs - non-voting
 Bernice Man
Bernice Man
Chairman, New Practitioners Network
(773) 702-9641
bernice.man.pharmd@gmail.com
 Abby Kahaleh
Abby Kahaleh
Ambulatory Care Network Chair
akahaleh@roosevelt.edu
 David Tjhio
David Tjhio
Chairman, Committee on Technology
(816) 885-4649
david.tjhio@cerner.com
 Jennifer Phillips
Jennifer Phillips
Editor & Chairman KeePosted
Committee Chair,
Nominations Committee
(630) 515-7167
jphillips@midwestern.edu
 Milena McLaughlin
Milena McLaughlin
Assistant Editor, KeePosted News Journal
(630) 515-7293
mmclau@midwestern.edu
Student Chapter Presidents
Ashley Shinnick - Chicago State University College of Pharmacy
Maggie Lau - Midwestern University Chicago College of Pharmacy
Kimberly Zaleski - Roosevelt University College of Pharmacy
Aprille Banchoencharoensuk - Rosalind Franklin University
College of Pharmacy
aprille.banchoencharoensuk@my.rfums.org
Kaylee Poole - Southern Illinois University Edwardsville School of Pharmacy
David Silva - University of Illinois at Chicago College of Pharmacy
HyeRim Whang Kong - University of Illinois at Chicago - Rockford Campus College of Pharmacy
ICHP Affiliates
Northern Illinois Society (NISHP)
Erika Hellenbart
President
Denise Kolanczyk
President-elect
Antoine Jenkins
Immediate Past President
(773) 821-2592
David Martin
Treasurer
Milena McLaughin
Secretary
(630) 515-7293
Vera Kalin
Technician Representative
West Central Society of Health-System Pharmacists (WSHP
Ed Rainville
President
ed.c.rainville@osfhealthcare.org
Metro East Society (MESHP)
Jared Sheley
President
Sangamiss Society of Heath-System Pharmacists
Julie Downen
President
217) 788-3953
Billee Samples
President-elect
Katelyn Conklen
Immediate Past President
Vacant Roles at Affiliates; President, Rock Valley Society; Southern IL Society; Sugar Creek Society

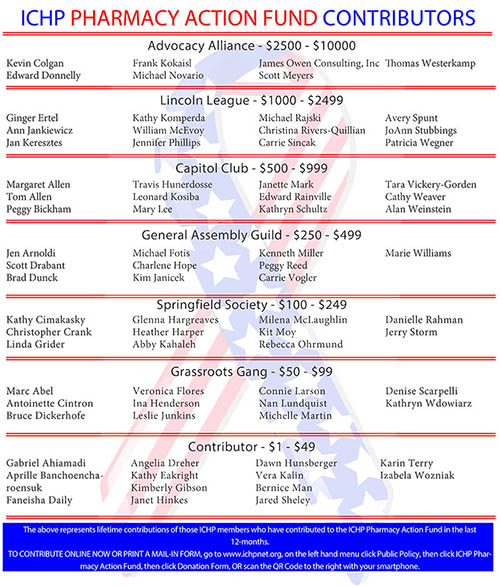
 Jennifer Arnoldi
Jennifer Arnoldi Scott Meyers
Scott Meyers Christopher Crank
Christopher Crank Clara Gary
Clara Gary