Print This Article
Educational Affairs
Updates in the Management of Persons with Diabetes and Chronic Kidney Disease
by Jessica Behnam, PharmD Candidate 2021, University of Illinois at Chicago College of Pharmacy; Myesha Tabriz, PharmD Candidate 2021, University of Illinois at Chicago College of Pharmacy; John Shilka, PharmD, BCPS, BCACP Clinical Pharmacist, Clinical Assistant Professor; University of Illinois at Chicago College of Pharmacy; Elizabeth Van Dril, PharmD, BCPS, BCACP; Clinical Assistant Professor, Department of Pharmacy Practice; University of Illinois at Chicago College of Pharmacy
Chronic kidney disease (CKD) and diabetes mellitus are two disease states which require timely evidence-based lifestyle and pharmacologic interventions for favorable patient outcomes. CKD is defined as an elevated urine albumin excretion (≥30 mg/g creatinine), and/or reduced estimated glomerular filtration rate (eGFR) below 60 ml/min/1.73m2 for greater than 3 months.1,2 Diabetes, one of the leading causes of CKD, induces inflammation and neurohormonal activation leading to glomerular hypertension and hyperfiltration.3,4 These pathophysiological processes may lead to albuminuria and/or progressively declining kidney function, which may ultimately result in end stage kidney disease (ESKD) if left untreated.4
There are several evidence-based management strategies for persons with diabetes and CKD. Recommendations include maintaining a healthy weight, eating a well-balanced diet, engaging in regular physical activity, promoting smoking cessation, and avoiding nephrotoxic agents.1,2 Of note, limiting dietary protein intake to below the recommended daily allowance of 0.8 g/kg/day is no longer recommended in persons with diabetes and nondialysis-dependent CKD, as it does not affect glycemic management, cardiovascular risk, or the rate of CKD progression.1 Treatment with a renin-angiotensin-aldosterone system (RAAS) inhibitor, such as an angiotensin converting enzyme inhibitor (ACEI) or angiotensin II receptor blocker (ARB), in persons with diabetes and hypertension, when albuminuria or reduced eGFR is present, is recommended to reduce CKD progression.1,2,5 In their 2020 Clinical Practice Guideline for Diabetes Management in CKD, Kidney Disease: Improving Global Outcomes (KDIGO) specifically recommends ACEIs and ARBs are titrated to the highest dose tolerated.2
There has been growing evidence to support the use of sodium-glucose cotransporter-2 (SGLT2) inhibitors in persons with type 2 diabetes (T2D) and CKD, despite lower antihyperglycemic efficacy in persons with a reduced eGFR (Table 1). SGLT2 inhibitors lower the renal threshold for glucose reabsorption by inhibiting SGLT2 in the proximal convoluted tubule, the primary site of glucose reabsorption, thus promoting glucosuria and improving glycemic management.4 Additionally, SGLT2 inhibitors are proposed to provide nephroprotection by reducing hyperfiltration and associated albuminuria. This may be derived from increased sodium excretion detected at the macula densa, which facilitates vasoconstriction of the afferent arteriole and reduced intraglomerular pressure via tubuloglomerular feedback. SGLT2 inhibitors induce a transient eGFR decline during the first weeks of treatment, which gradually returns to baseline and then stabilizes - implicating a long-term nephroprotective effect.
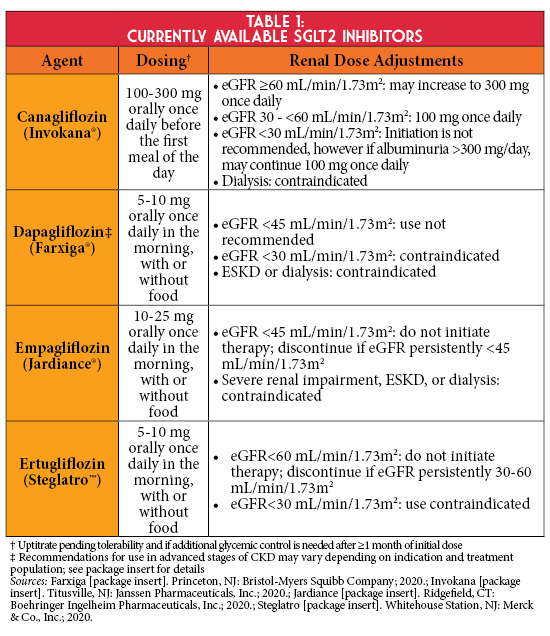
In cardiovascular outcomes trials (CVOTs), empagliflozin, canagliflozin, and dapagliflozin significantly reduced the risk for secondary renal outcomes.1 Findings from these landmark trials have laid the foundation for more recent renal outcome studies to assess the effect of SGLT2 inhibitors on a primary renal endpoint (Table 2). The CREDENCE trial demonstrated that in persons with albuminuric diabetic kidney disease, canagliflozin significantly reduced the risk of kidney failure and cardiovascular events compared to placebo.6 Results of this trial led to updated prescribing information for canagliflozin, allowing its initiation in Stage 3b CKD (eGFR 30-44 mL/min/1.73m2) if albuminuria is present and permits continuation as eGFR declines until dialysis or transplantation.7 More recently, the DAPA-CKD trial showed dapagliflozin significantly reduced the risk of the composite endpoint of a sustained decline in eGFR of at least 50%, ESKD, or death from renal or cardiovascular causes in patients with CKD irrespective of diabetes status.8 Renal outcome data in patients with advanced CKD without albuminuria are anticipated with the completion of the EMPA-KIDNEY trial, which is studying empagliflozin in this population.9
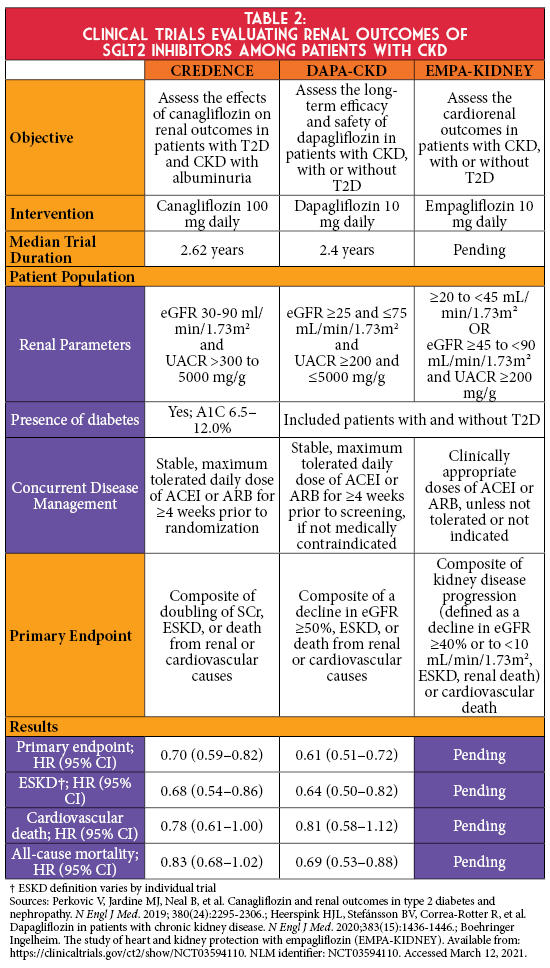
Collective findings from these trials have reshaped clinical guidelines for the management of persons with T2D and CKD. While the KDIGO and American Diabetes Association (ADA) guidelines continue to recommend metformin first-line for persons with T2D and CKD, they now recommend considering the addition of a SGLT2 inhibitor irrespective of baseline A1C or treatment with metformin.1,2 Both guidelines recommend SGLT2 inhibitor initiation when eGFR is ≥30 mL/min/1.73m2, and KDIGO further recommends that these agents can be continued if eGFR subsequently declines until initiation of kidney replacement therapy.2 The ADA preferences using SGLT2 inhibitors that have evidence of reducing CKD progression in populations studied in renal outcome trials (i.e., canagliflozin and dapagliflozin) and to consider SGLT2 inhibitors with evidence of reducing secondary renal outcomes in CVOTs.1
Before initiating a SGLT2 inhibitor, it is essential to consider background antihyperglycemic therapy. For example, reducing the dose or discontinuing an antihyperglycemic medication with risk for hypoglycemia (i.e., insulin or secretagogues) may be needed to safely initiate these agents. It is also important to monitor eGFR at baseline and periodically thereafter with the initiation of a SGLT2 inhibitor, while noting that the transient decrease in eGFR may not necessitate therapy discontinuation. In addition to concurrent antihyperglycemic therapy, it is also important to evaluate medications that impact volume status. Decreasing thiazide or loop diuretic doses may be considered before SGLT2 inhibitor initiation to mitigate hypovolemia risk when used concurrently; however, no formal dosing guidance exists with this practice.
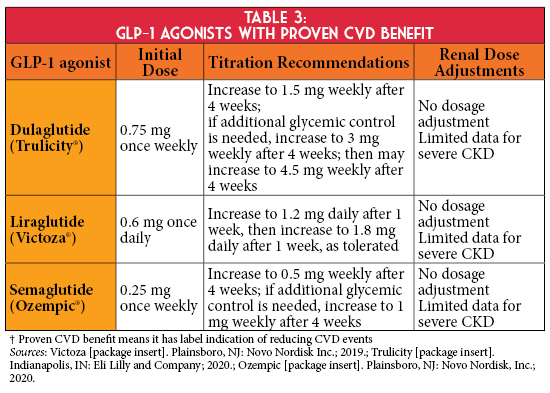
If individualized glycemic targets are not achieved despite the use of metformin and a SGLT2 inhibitor, or if a SGLT2 inhibitor is not tolerated or contraindicated, KDIGO and ADA guidelines recommend using a GLP-1 agonist with proven cardiovascular disease (CVD) benefit,1,2 as the primary cause of death in persons with CKD is CVD (Table 3).3 Therefore, the use of select GLP-1 agonists in this patient population may be beneficial given their cardiovascular risk reduction. In addition, the SUSTAIN 6 trial demonstrated semaglutide was associated with lower rates of new or worsening nephropathy.10 Currently, no trial has evaluated renal outcomes with GLP-1 agonists in a CKD population; however, the FLOW trial is ongoing to assess the effect of semaglutide in patients with T2D and CKD on its primary composite renal outcome.11
Additionally, a novel nonsteroidal mineralocorticoid antagonist, finerenone, was recently evaluated to determine its effect on kidney disease progression in the FIDELIO-DKD trial.12 Treatment with finerenone resulted in a lower risk of CKD progression and adverse cardiovascular events compared to placebo in persons with T2D and advanced CKD. The findings of this trial have not been incorporated in the KDIGO guideline; however, this trial introduced an additional therapeutic option for this population and laid the foundation for another trial, FIGARO-DKD, to explore the effects of finerenone on cardiovascular morbidity and mortality in patients with less advanced CKD.13
In conclusion, a comprehensive approach should be utilized when managing persons with CKD and T2D to reduce risks of kidney disease progression and CVD. Large outcomes trials have demonstrated the nephroprotective benefits of select SGLT2 inhibitors in this population and thus are recommended by KDIGO and ADA guidelines irrespective of baseline A1C or metformin treatment status. Ongoing outcomes trials for both SGLT2 inhibitors and GLP-1 agonists may provide further clarification for their role in this population, while finerenone represents a novel and promising therapy option for a disease state in which limited pharmacotherapeutic agents exist to slow its progression.
References:
- American Diabetes Association. Standards of Medical Care in Diabetes - 2021. Diabetes Care. 2021;44(Suppl.1):S1-232.
- Kidney Disease: Improving Global Outcomes (KDIGO) Diabetes Work Group. KDIGO 2020 Clinical practice guideline for diabetes management in chronic kidney disease. Kidney Int. 2020;98(4S):S1–S115.
- United States Renal Data System. 2020 USRDS Annual Data Report: Epidemiology of kidney disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD, 2020.
- van Bommel EJ, Muskiet MH, Tonneijck L, et al. SGLT2 inhibition in the diabetic kidney-from mechanisms to clinical outcome. Clin J Am Soc Nephrol. 2017;12(4):700-710.
- Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018;71(6):e127-248.
- Perkovic V, Jardine MJ, Neal B, et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019; 380(24):2295-2306.
- Invokana [package insert]. Titusville, NJ: Janssen Pharmaceuticals, Inc.; 2020.
- Heerspink HJL, Stefánsson BV, Correa-Rotter R, et al. Dapagliflozin in patients with chronic kidney disease. N Engl J Med. 2020;383(15):1436-1446.
- Boehringer Ingelheim. The study of heart and kidney protection with empagliflozin (EMPA-KIDNEY). Available from: https://clinicaltrials.gov/ct2/show/NCT03594110. NLM identifier: NCT03594110. Accessed March 12, 2021.
- Marso SP, Bain SC, Consoli A, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375(19):1834-1844.
- Novo Nordisk A/S. A research study to see how semaglutide works compared to placebo in people with type 2 diabetes and chronic kidney disease (FLOW). Available from: https://clinicaltrials.gov/ct2/show/NCT03819153. NLM identifier: NCT03819153. Accessed March 12, 2021.
- Bakris GL, Agarwal R, Anker SD, et al. Effect of finerenone on chronic kidney disease outcomes in type 2 diabetes. N Engl J Med. 2020;383(23):2219-2229.
- ClinicalTrials.gov. Efficacy and safety of finerenone in subjects with type 2 diabetes mellitus and the clinical diagnosis of diabetic kidney disease (FIGARO-DKD). Available from: https://clinicaltrials.gov/ct2/show/NCT02545049. NLM identifier: NCT02545049. Accessed March 12, 2021.





