Official Newsjournal of the Illinois Council of Health-System Pharmacists
Educational Affairs
Blast from the Past: Convalescent Plasma for COVID-19
by Katie Miles, PharmD Clinical Assistant Professor/Academic Detailing Pharmacist; University of Chicago College of Pharmacy; Heather Ipema, PharmD, BCPS Clinical Assistant Professor; Drug Information Group; University of Chicago College of Pharmacy
At this time, there are no Food and Drug Administration (FDA)-approved treatment options for COVID-19 and no medication has been shown to be safe and effective for this infection.4,5 One potential treatment that has been used and is regulated by the FDA as an investigational product is COVID-19 convalescent plasma (herein after referred to as “convalescent plasma”).5,6 The Infectious Diseases Society of America (IDSA) recommends convalescent plasma in hospitalized patients in the setting of a clinical trial; though, the authors note a knowledge gap for this recommendation.2 The National Institutes of Health (NIH) also address convalescent plasma in their COVID-19 treatment guideline, but are unable to recommend for or against its use (graded as a strong recommendation based on expert opinion [AIII]) due to a lack of data and theoretical risks (e.g., antibody-dependent enhancement of infection and transfusion-associated lung injury [TRALI]).4
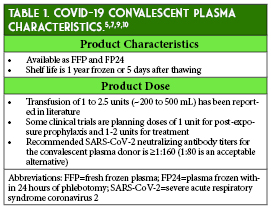
The dose and preferred timing for administration of convalescent plasma during the course of a COVID-19 infection has not been established.7 Dosing of convalescent plasma in general has been widely variable throughout the history of its use as post-exposure prophylaxis and/or treatment in several viral infections (e.g., polio and Ebola), including respiratory infection outbreaks, including the 2009-2010 H1N1 influenza virus pandemic, 2003 SARS-CoV-1 epidemic, and 2012 Middle East Respiratory Syndrome coronavirus (MERS-CoV) epidemic.5,9 Once convalescent plasma is administered, the duration of the circulating antibodies’ therapeutic effect is unclear and dependent on the antibody amount given, but may last weeks to months.8,9 Table 1 provides additional characteristics of COVID-19 convalescent plasma.
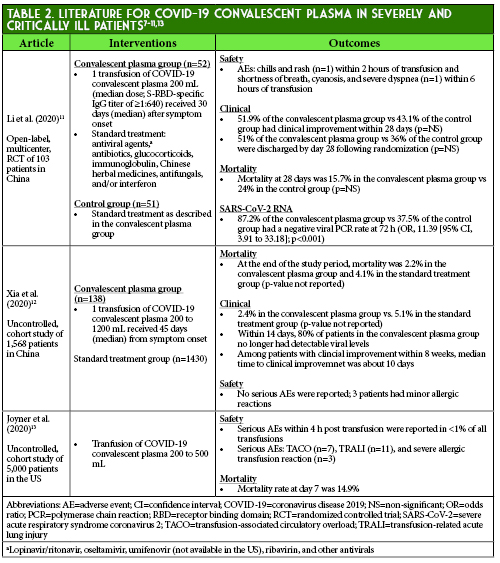
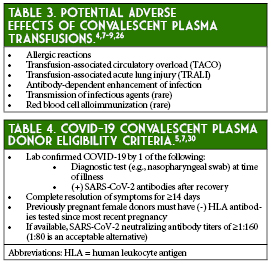
Convalescent plasma transfusion has several theoretical risks associated with its use based on known risks associated with standard plasma transfusions that are therefore not unique to this specific product (Table 3).4,7-9, 26 These potential adverse effects have not been fully established for COVID-19 convalescent plasma.7 The historical data from SARS and MERS suggest that convalescent plasma use in coronavirus infections is potentially safe, although two case reports of possible convalescent plasma-induced TRALI events have been described in a patient with Ebola and a patient with MERS.8,9, 26-28 Possible transfusion-related adverse events that resolved with corticosteroid treatment were reported in two patients in the randomized controlled trial by Li et al.11 Additionally, Joyner et al. reported limited cases of transfusion-associated circulatory overload (TACO), TRALI, and severe allergic reactions, but no significant adverse events were reported in most of the COVID-19 studies or in several previous SARS-associated coronavirus and severe influenza studies.13, 14, 16-22, 29
- Fauci AS, Lane HC, Redfield RR. Covid-19 - navigating the uncharted. N Engl J Med. 2020;382:1268-1269.
- Bhimraj A, Morgan RL, Shumaker AH, et al. Infectious Diseases Society of America guidelines on the treatment and management of patients with COVID-19. Clin Infect Dis. 2020.
- World Health Organization. WHO director-general's opening remarks at the media briefing on COVID-19 - 11 March 2020. https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020 (accessed 2020 July 10).
- National Institutes of Health. COVID-19 treatment guidelines (updated May 12, 2020). https://covid19treatmentguidelines.nih.gov/therapeutic-options-under-investigation/host-modifiers-immunotherapy/ (accessed 2020 July 10).
- Food and Drug Administration. Recommendations for investigational COVID-19 convalescent plasma (updated May 1, 2020). https://www.fda.gov/vaccines-blood-biologics/investigational-new-drug-ind-or-device-exemption-ide-process-cber/recommendations-investigational-covid-19-convalescent-plasma (accessed 2020 July 10).
- Food and Drug Administration. Coronavirus disease 2019 (COVID-19) resources for health professionals (updated July 6, 2020). https://www.fda.gov/health-professionals/coronavirus-disease-2019-covid-19-resources-health-professionals (accessed 2020 July 10).
- American Society of Health-System Pharmacists. Assessment of evidence for COVID-19-related treatments (updated July 2, 2020). https://www.ashp.org/-/media/assets/pharmacy-practice/resource-centers/Coronavirus/docs/ASHP-COVID-19-Evidence-Table.ashx (accessed 2020 July 10).
- Casadevall A, Pirofski LA. The convalescent sera option for containing COVID-19. J Clin Invest. 2020;130:1545-1548.
- Bloch EM, Shoham S, Casadevall A, et al. Deployment of convalescent plasma for the prevention and treatment of COVID-19. J Clin Invest. 2020.
- American Red Cross. Coronavirus (COVID-19) convalescent plasma clinician information. https://www.redcrossblood.org/donate-blood/dlp/plasma-donations-from-recovered-covid-19-patients/clinician-registration.html (accessed 2020 July 10).
- Li L, Zhang W, Hu Y, et al. Effect of convalescent plasma therapy on time to clinical improvement in patients with severe and life-threatening COVID-19: a randomized clinical trial. JAMA. 2020.
- Xia X, Kening L, Lingxiang W, et al. Improved clinical symptoms and mortality on severe/critical COVID-19 patients utilizing convalescent plasma transfusion. Blood. 2020.
- Joyner M, Wright RS, Fairweather D, et al. Early safety indicators of COVID-19 convalescent plasma in 5,000 patients. medRxiv. 2020.
- Mehmet AE, Sarici A, Berber I, et al. Life-saving effect of convalescent plasma treatment in COVID-19 disease: clinical trial from eastern Anatolia. Transfus Apher Sci. 2020.
- Hartman W, Hess AS, Connor JP. Hospitalized COVID-19 patients treated with convalescent plasma in a mid-size city in the midwest. medRxiv. 2020.
- Hegerova L, Gooley T, Sweerus KA, et al. Use of convalescent plasma in hospitalized patients with COVID-19 – case series. Blood. 2020.
- Salazar E, Perez KK, Ashraf M, et al. Treatment of COVID-19 patients with convalescent plasma. Am J Pathol. 2020.
- Olivares-Gazca JC, Priesca-Marin JM, Ojeda-Laguna M, et al. Infusion of convalescent plasma is associated with clincial improvement in critically ill patients with COVID-19: a pilot study. Rev Invest Clin. 2020;72:159-164.
- Liu STH, Lin H, Baine I, et al. Convalescent plasma treatment of severe COVID-19: a matched control study. medRxiv. 2020.
- Gharbharan A, Jordans CCE, GeurtsvanKessel C, et al. Convalescent plasma for COVID-19. A randomized clinical trial. medRxiv. 2020.
- Duan K, Liu B, Li C, et al. Effectiveness of convalescent plasma therapy in severe COVID-19 patients. Proc Natl Acad Sci U S A. 2020;117:9490-9496.
- Zeng QL, Yu ZJ, Gou JJ, et al. Effect of convalescent plasma therapy on viral shedding and survival in COVID-19 patients. J Infect Dis. 2020.
- Valk SJ, Piechotta V, Chai KL, et al. Convalescent plasma or hyperimmune immunoglobulin for people with COVID-19: a rapid review. Cochrane Database Syst Rev. 2020;5:Cd013600.
- Roback JD, Guarner J. Convalescent plasma to treat COVID-19: possibilities and challenges. JAMA. 2020.
- Casadevall A, Joyner MJ, Pirofski LA. A randomized trial of convalescent plasma for COVID-19-potentially hopeful signals. JAMA. 2020.
- Tiberghien P, de Lamballerie X, Morel P, et al. Collecting and evaluating convalescent plasma for COVID-19 treatment: why and how? Vox Sang. 2020.
- Chun S, Chung CR, Ha YE, et al. Possible transfusion-related acute lung injury following convalescent plasma transfusion in a patient with Middle East Respiratory Syndrome. Ann Lab Med. 2016;36:393-395.
- Mora-Rillo M, Arsuaga M, Ramírez-Olivencia G, et al. Acute respiratory distress syndrome after convalescent plasma use: treatment of a patient with Ebola virus disease contracted in Madrid, Spain. Lancet Respir Med. 2015;3:554-562.
- Mair-Jenkins J, Saavedra-Campos M, Baillie JK, et al. The effectiveness of convalescent plasma and hyperimmune immunoglobulin for the treatment of severe acute respiratory infections of viral etiology: a systematic review and exploratory meta-analysis. J Infect Dis. 2015;211(1):80-90.
- Food and Drug Administration. Guidance for industry: investigational COVID-19 convalescent plasma (May 26, 2020). https://www.fda.gov/regulatory-information/search-fda-guidance-documents/investigational-covid-19-convalescent-plasma (accessed 2020 July 10).
- Mayo Clinic. COVID-19 expanded access program. https://www.uscovidplasma.org/ (accessed 2020 July 10).
- Mayo Clinic. How do individuals donate plasma for this protocol? https://www.uscovidplasma.org/donate (accessed 2020 July 10).
- American Society of Hematology. COVID-19 and convalescent plasma: frequently asked questions (updated June 8, 2020). https://www.hematology.org/covid-19/covid-19-and-convalescent-plasma (accessed 2020 July 10).
Contents
Columns
There's Still Time to Step Up!
We Need Reasons to Celebrate This Year!
Features
Opioid Task Force - CPE Opportunity!
College Connection
Midwestern University Chicago College of Pharmacy
Roosevelt University College of Pharmacy
Rosalind Franklin University College of Pharmacy
Southern Illinois University Edwardsville School of Pharmacy
More
Pharmacy Action Fund Contributors
ICHP Board of Directors 2020 - 2021
Regularly Scheduled Network Meetings
Chicago Area Pharmacy Directors Network Dinner
3rd Thursday of Odd Months
5:30pm
Regularly Scheduled Division and Committee Calls
Executive Committee
Second Tuesday of each month at 7:00 p.m.
Educational Affairs
Third Tuesday of each month at 11:00 a.m.
Government Affairs
Third Monday of each month at 5:00 p.m.
Marketing Affairs
Third Tuesday of each month at 8:00 a.m.
Organizational Affairs
Fourth Thursday of each month at 12:00 p.m.
Professional Affairs
Fourth Thursday of each month at 2:00 p.m.
New Practitioner Network
Second Thursday of each month at 5:30 p.m.
Technology Committee
Second Friday of each month at 8:00 a.m.
Chicago Area Pharmacy Directors Network Dinner
Bi-monthly in odd numbered months with dates to be determined. Invitation only.
