Official Newsjournal of the Illinois Council of Health-System Pharmacists
Educational Affairs
Post-Pandemic Prevention: The Development of a COVID-19 Vaccine
by Jason Val G. Alegro, PharmD, BCPS, BCIDP; Clinical Pharmacy Specialist, Infectious Diseases, Mount Sinai Hospital & Assistant Professor of Clinical Sciences, Roosevelt University College of Pharmacy; Chelsea Manaligod, PharmD Candidate Roosevelt University College of Pharmacy; Kristen Martinez, PharmD Candidate Roosevelt University College of Pharmacy
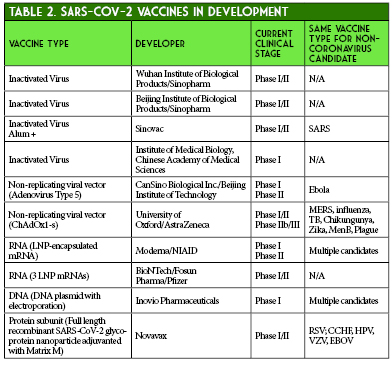
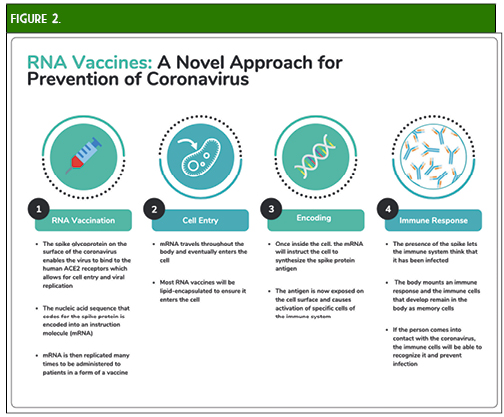
Moderna provided preliminary data on May 18, 2020. The press release provided immunogenicity data for participants receiving two doses of 25 mcg or 100 mcg and only after the first dose of 250 mcg (n = 15 in each cohort). All subjects achieved seroconversion after one dose, and for the 25 mcg and 100 mcg doses, binding antibody levels were similar to those seen in convalescent sera. Eight participants in the 25 mcg and 100 mcg cohorts achieved serum neutralizing antibody titers at or above levels seen in convalescent sera. Three patients in the 250 mcg cohort experienced grade 3 adverse events after the second dose, which were transient and self-resolving. One grade 3 adverse event of erythema around the injection site occurred in one participant in the 100 mcg group. Zero adverse events occurred in participants in the 25 mcg cohort.29 The mRNA-1273 vaccine is currently undergoing Phase II trials comparing 50 mcg and 100 mcg doses with an estimated enrollment of 600 participants.30 A recent press release from Moderna stated that Phase III trials are expected to begin in July and that the study protocol has been finalized. The trial will be a randomized 1:1 (100 mcg dose vs. placebo) trial with an expected enrollment of 30,000 United States participants and a primary endpoint of the prevention of symptomatic COVID-19 infection.31
There are many questions regarding these vaccines that will need to be addressed through clinical trials and long-term follow-up. What is the vaccine effectiveness and durability? Are there any concerns for short and long-term safety particularly with newer vaccine technologies? With the variability seen in patient responses to COVID-19, how will this translate to prevention variability among different demographics, especially in the elderly? How will the vaccine overcome antigenic drift and shift of SARS-CoV-2? What will the role of herd immunity and vaccination rates play in preventing COVID-19 in the future? The successful availability, stability, and distribution of these vaccines will preclude many of these questions. It will only be once a successful vaccine is approved and marketed that we will be able to see its true effect on the population, hopefully leading to an effective post-pandemic reality. ■
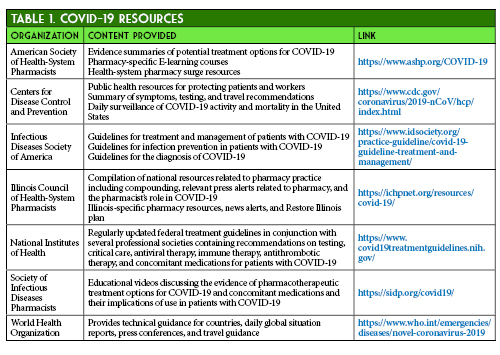
- American Society of Health System Pharmacists. ASHP COVID-19 Resource Center. https://www.ashp.org/COVID-19 (accessed 2020 Jun 12)
- Centers for Disease Control and Prevention. Information for Healthcare Professionals about Coronavirus (COVID-19). https://www.cdc.gov/coronavirus/2019-nCoV/hcp/index.html (accessed 2020 Jun 12)
- Infectious Diseases Society of America. Infectious Diseases Society of America Guidelines on the Treatment and Management of Patients with COVID-19. https://www.idsociety.org/practice-guideline/covid-19-guideline-treatment-and-management/ (accessed 2020 Jun 12)
- Illinois Council of Health-System Pharmacists. ICHP COVID-19 Resources. https://ichpnet.org/resources/covid-19/ (accessed 2020 Jun 12)
- National Institutes of Health. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. https://www.covid19treatmentguidelines.nih.gov/ (accessed 2020 Jun 12)
- Society of Infectious Diseases Pharmacists. COVID-19 Resources. http://sidp.org/covid19/ (accessed 2020 Jun 12)
- World Health Organization. Coronavirus disease (COVID-19) pandemic. https://www.who.int/emergencies/diseases/novel-coronavirus-2019 (accessed 2020 Jun 12)
- Thometz K. Pritzker Says Illinois on Track to Move to Phase 4, Won’t Lift Restrictions Early. Wttw. 6/10/20.
- Restore Illinois: A Public Health Approach to Safely Reopen Our State. Office of JB Pritzker. https://coronavirus.illinois.gov/sfc/servlet.shepherd/document/download/069t000000BadS0AAJ?operationContext=S1 (accessed 2020 Jun 12).
- Brennan Z, Goldberg D. Coronavirus drugmakers' latest tactics: Science by press release. Politico. 6/8/20.
- Jin Y, Yang H, Ji W, et al. Virology, Epidemiology, Pathogenesis, and Control of COVID-19. Viruses. 2020;12(4):372. Published 2020 Mar 27. doi:10.3390/v12040372,
- https://it.wikipedia.org/wiki/File:3D_medical_animation_corona_virus.jpg
- Zhang H, Penninger, JM, Li Y, et al. Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: molecular mechanisms and potential therapeutic target. Intensive Care Med. 46: 586–590. doi: 10.1007/s00134-020-05985-9
- Zheng M, Song L. Novel antibody epitopes dominate the antigenicity of spike glycoprotein in SARS-CoV-2 compared to SARS-CoV. Cell Mol Immunol. 2020. 17; 536-538. https://doi.org/10.1038/s41423-020-0385-z
- Prekumar L, Segovia-Chumbez B, Jadi R, Martinez D, Raut R. The receptor binding domain of the viral spike protein is an immunodominant and highly specific target of antibodies in SARS-CoV-2 patients. Science Immunology. 2020; 5: 1-14. DOI: 10.1126/sciimmunol.abc8413
- Mukherjee S, Tworowski D, Detroja R, Mukherjee SB, Frenkel-Morgenstern M. Immunoinformatics and Structural Analysis for Identification of Immunodominant Epitopes in SARS-CoV-2 as Potential Vaccine Targets.Vaccines (Basel). 2020. E290; 1-17. doi: 10.3390/vaccines8020290.
- Ju B, Zhang Q, Ge J, et al. Human neutralizing antibodies elicited by SARS-CoV-2 infection [published online ahead of print, 2020 May 26]. Nature. 2020;10.1038/s41586-020-2380-z. doi:10.1038/s41586-020-2380-z
- Callaway E. The race for coronavirus vaccines: a graphical guide. Nature. 2020;580(7805):576‐577. doi:10.1038/d41586-020-01221-y
- World Health Organization. DRAFT landscape of COVID-19 candidate vaccines–30 April 2020. https://www.who.int/who-documents-detail/draft-landscape-of-covid-19-candidate-vaccines. (accessed 2020 June 13)
- A Phase II Clinical Trial to Evaluate the Recombinant Vaccine for COVID-19 (Adenovirus Vector) (CTII-nCoV). ClinicalTrials.gov Identifier: NCT04341389. https://clinicaltrials.gov/ct2/show/NCT04341389
- Amanat F, Krammer F. SARS-CoV-2 Vaccines: Status Report. Immunity. 2020;52(4):583‐589. doi:10.1016/j.immuni.2020.03.007
- Robert-Guroff M. Replicating and non-replicating viral vectors for vaccine development. Curr Opin Biotechnol. 2007;18(6):546‐556. doi:10.1016/j.copbio.2007.10.010
- Ahi YS, Bangari DS, Mittal SK. Adenoviral vector immunity: its implications and circumvention strategies. Curr Gene Ther. 2011;11(4):307‐320. doi:10.2174/156652311796150372
- Zhu FC, Li YH, Guan XH, et al. Safety, tolerability, and immunogenicity of a recombinant adenovirus type-5 vectored COVID-19 vaccine: a dose-escalation, open-label, non-randomised, first-in-human trial. Lancet. 2020; 395: 1845-1854.
- Safety and immunogenicity study of 2019-nCoV Vaccine (mRNA-1273) to prevent SARS-CoV-2 Infection. ClinicalTrials.gov Identifier: NCT04283461. https://clinicaltrials.gov/ct2/show/NCT04283461
- Zhang C, Maruggi G, Shan H, Li J. Advances in mRNA Vaccines for Infectious Diseases. Front Immunol. 2019; 10: 1-13.
- Wang F, Kream RM, Stefano GB. An Evidence Based Perspective on mRNA-SARS-CoV-2 Vaccine Development. Med Sci Monit. 2020;26:e924700. Published 2020 May 5. doi:10.12659/MSM.924700
- Diamond MS, Pierson TC. The Challenges of Vaccine Development against a New Virus during a Pandemic. Cell Host Microbe. 2020;27(5):699‐703. doi:10.1016/j.chom.2020.04.021
- Moderna Announces Positive Interim Phase 1 Data For Its Mrna Vaccine (Mrna-1273) Against Novel Coronavirus. May 18, 2020. Press release.
- Dose-Confirmation Study to Evaluate the Safety, Reactogenicity, and Immunogenicity of mRNA-1273 COVID-19 Vaccine in Adults Aged 18 Years and Older. Clinicaltrials.gov Identifier: NCT04405076.
- Moderna Advances Late-Stage Development of its Vaccine (mRNA-1273) Against COVID-19. June 11, 2020. Press release.
Contents
Columns
There's Still Time to Step Up!
We Need Reasons to Celebrate This Year!
Features
Opioid Task Force - CPE Opportunity!
College Connection
Midwestern University Chicago College of Pharmacy
Roosevelt University College of Pharmacy
Rosalind Franklin University College of Pharmacy
Southern Illinois University Edwardsville School of Pharmacy
More
Pharmacy Action Fund Contributors
ICHP Board of Directors 2020 - 2021
Regularly Scheduled Network Meetings
Chicago Area Pharmacy Directors Network Dinner
3rd Thursday of Odd Months
5:30pm
Regularly Scheduled Division and Committee Calls
Executive Committee
Second Tuesday of each month at 7:00 p.m.
Educational Affairs
Third Tuesday of each month at 11:00 a.m.
Government Affairs
Third Monday of each month at 5:00 p.m.
Marketing Affairs
Third Tuesday of each month at 8:00 a.m.
Organizational Affairs
Fourth Thursday of each month at 12:00 p.m.
Professional Affairs
Fourth Thursday of each month at 2:00 p.m.
New Practitioner Network
Second Thursday of each month at 5:30 p.m.
Technology Committee
Second Friday of each month at 8:00 a.m.
Chicago Area Pharmacy Directors Network Dinner
Bi-monthly in odd numbered months with dates to be determined. Invitation only.
