Official Newsjournal of the Illinois Council of Health-System Pharmacists
Professional Affairs
Pharmacist-Led Implementation of a Direct Oral Anticoagulant Prescribing Guideline and Evaluation of Prescribing Practices at a Community Teaching Hospital
by Paula Bielnicka, PharmD; Clinical Staff Pharmacist & Outpatient Anticoagulation Pharmacist, Swedish Hospital, Part of NorthShore, Chicago, IL and Alicia Juska, PharmD, BCPS; Director of Pharmacy Services/Residency Program Director, Swedish Hospital, Part of NorthShore, Chicago, IL and John Shilka, PharmD, BCPS; Clinical Pharmacist, Managed Care/Internal Medicine, University of Illinois at Chicago - College of Pharmacy, Chicago, IL
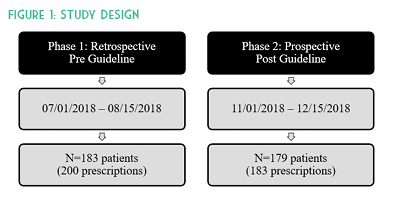
Creation of a hospital formulary-specific DOAC guideline standardizing rivaroxaban and apixaban doses per anticoagulation indication and duration was approved by the Pharmacy & Therapeutics Committee and the project was reviewed by an Institutional Review Board. Outpatient prescriptions were not evaluated. Patients were eligible for inclusion in this study if they were treated with apixaban or rivaroxaban, were at least 18 years of age, and were not pregnant. The primary outcome measured the rate of appropriate inpatient prescriptions concordant with package insert labeling. Inappropriate prescriptions were classified as either an overdose or under-dose to determine where healthcare provider education was needed. Secondary outcomes included number of thrombotic or bleeding events during hospitalization, readmissions for bleeds/thromboembolisms, and patients with end stage renal disease.
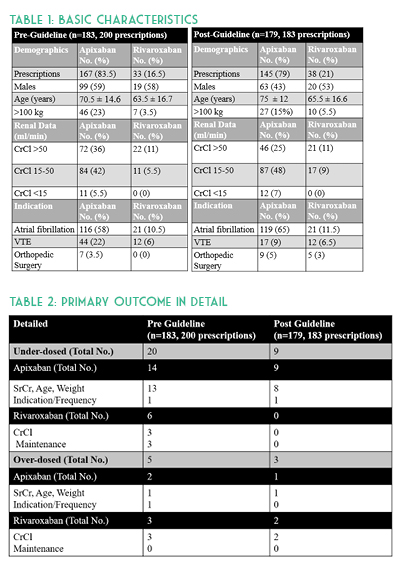
A total of 383 orders were included with 200 prescriptions in the pre-guideline arm and 183 prescriptions in the post-guideline arm. Baseline characteristics were similar in age, anticoagulation indication, and about half of all patients in each group had a creatinine clearance of 15 to 50 mL/min. Overall there were more males in the pre-guideline arm (64% versus 46%) and approximately 24% of all patients were above100 kg. No p-values were calculated for baseline characteristics (See Table 1). DOACs were appropriately dosed in 87% (175/200) versus 93% (171/183) of prescriptions pre- and post-guideline implementation, respectively. Evaluation of inappropriate apixaban prescriptions alone identified 16 (10%) pre-guideline compared with 10 (7%) post-guideline. Assessment of rivaroxaban dosing alone identified nine (27%) inappropriate pre-guideline doses compared to two (5%) post-guideline doses. After investigating the 37 inappropriate hospital DOAC prescriptions, it was determined that more than half were due to continuation of a patient’s original home dose upon hospital admission. Missed opportunities were due to both pharmacist and provider hesitation to change established home dosages and from providers denying auto substitutions. (See Table 2.)
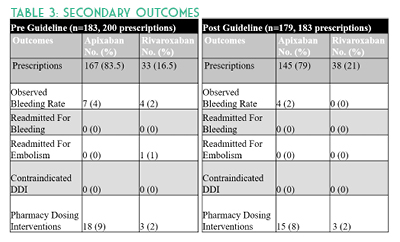
Study strengths included use of an electronic medical record (EMR) system which contains medication detail, including patients’ external fill history, patient specific information, prescriber documentation, and laboratory results. This allowed for more accurate assessment of dosing appropriateness and potential for drug-drug interactions. Implementation of pharmacist-led renal dose adjustments allowed for timely administration of appropriate doses based on daily hospital laboratory results. Study limitations include a small sample size and timeframe compared to previously published DOAC studies.4-6 Some patient information was missing at time of inpatient order verification, which was later discovered during the visit. For instance, anticoagulation indication and appropriateness were unclear for AF patients hospitalized for another indication such as DVT. These occurrences were not excluded, but rather the newest diagnosis was evaluated as the primary anticoagulation indication in the retrospective data collection. Another limitation is that CHA2DS2-VASc scores were not calculated and therefore not used to determine anticoagulation appropriateness in AF patients. Discharge prescriptions were not evaluated in this study. Other barriers include potential drug-drug interactions due to unavailable external medication fill histories and incomplete medication histories. Future studies should evaluate the home DOAC regimen and appropriateness of continuing this on hospital admission as well as upon discharge.
Results suggest underdosing in apixaban patients, especially continuation of home doses upon admission, may require further provider education. As evidenced by this study, implementation of a pharmacist-led DOAC guideline allowing for renal dose adjustments may improve both FDA-approved concordant prescribing of these medications and decrease rates of bleeding.
- Eliquis (apixaban) package insert. Princeton, NJ: Bristol-Myers Squibb Company and Pfizer Inc.; 2015.
- Xarelto (rivaroxaban) package insert. Titusville, NJ: Janssen Pharmaceuticals, Inc.; 2012.
- Yao X, Shah ND, Sangaralingham LR, Gersh BJ, Noseworthy PA. Non-vitamin k antagonist oral anticoagulant dosing in patients with atrial fibrillation and renal dysfunction. J Am Coll Cardiol. 2017;69(23):2779-2790.
- Ruiz OM, Muniz J, Rana M, et al. Inappropriate doses of direct oral anticoagulants in real-world clinical practice: prevalence and associated factors. A subanalysis of the FANTASSIA Registry. Europace. 2018;20(10):1577-1583.
- McAlister FA, Garrison S, Kosowan L, Ezekowitz JA, Singer A. Use of direct oral anticoagulants in Canadian primary care practice 2010–2015: A cohort study from the Canadian primary care sentinel surveillance network. J Am Heart Assoc. 2018;7(3): e007603. https://www.ahajournals.org/doi/full/10.1161/JAHA.117.007603. (accessed 2018 May 27).
- Steinberg BA, Shrader P, Thomas L, et al. Off-label dosing of non-vitamin k antagonist oral anticoagulants and adverse outcomes: the ORBIT-AF II Registry. J Am Coll Cardiol. 2016;68(24):2597-2604.
Contents
Columns
Features
2020 Spring Meeting Poster Presentations
Opioid Task Force - CPE Opportunity!
College Connection
Midwestern University Chicago College of Pharmacy
Roosevelt University College of Pharmacy
Rosalind Franklin University of Medicine and Science College of Pharmacy
Southern Illinois University Edwardsville (SIUE) - School of Pharmacy
University of Illinois at Chicago College of Pharmacy
More
ICHP Pharmacy Action Fund (PAC)
Board of Directors, Student Society Presidents & Affiliates
Regularly Scheduled Network Meetings
Chicago Area Pharmacy Directors Network Dinner
3rd Thursday of Odd Months
5:30pm
Regularly Scheduled Division and Committee Calls
Executive Committee
Second Tuesday of each month at 7:00 p.m.
Educational Affairs
Third Tuesday of each month at 11:00 a.m.
Government Affairs
Third Monday of each month at 5:00 p.m.
Marketing Affairs
Third Tuesday of each month at 8:00 a.m.
Organizational Affairs
Fourth Thursday of each month at 12:00 p.m.
Professional Affairs
Fourth Thursday of each month at 2:00 p.m.
New Practitioner Network
Second Thursday of each month at 5:30 p.m.
Technology Committee
Second Friday of each month at 8:00 a.m.
Chicago Area Pharmacy Directors Network Dinner
Bi-monthly in odd numbered months with dates to be determined. Invitation only.
