Print This Article
Educational Affairs
Pharmacogenomics: A Brief Overview and Guide to Clinical and Educational Resources
by James C. Lee, PharmD, BCACP - Clinical Assistant Professor, Clinical Lead and Co-Director, UI Health Personalized Medicine Program - University of Illinois Hospital & Health Sciences System, University of Illinois at Chicago College of Pharmacy
Background
Precision medicine incorporates factors related to an individual’s genetic variation, lifestyle, and environment to guide the treatment, management, and prevention of disease.1 Pharmacists have already been applying tenets of precision medicine to individualize pharmacotherapy through incorporating clinical factors such as age, weight, sex, laboratory results, and organ function to guide the selection and dosing of medications. Contemporary medication selection and dosing, however, still largely follows a population-level, “one-size-fits-all”, trial-and-error approach necessitating multiple modifications that prolong the time to achieving optimal treatment outcomes while exposing patients to potential severe adverse drug reactions.
Of particular interest to pharmacy is the application of pharmacogenomics and individuals’ genetic data to truly individualize pharmacotherapy.2 Pharmacogenetics, a subset of pharmacogenomics, aims to elucidate the impact of individual genetic variability commonly arising from single nucleotide polymorphisms on drug pharmacokinetics and pharmacodynamics. The clinical applications of pharmacogenomics have largely focused on germline genetic variations (e.g., genetic variations inherited at conception), although somatic genetic variations (e.g., variations spontaneously occurring during an individual’s life) are also of increasing clinical and research interest in the field of oncology.
As of 2019, over 200 Food and Drug Administration (FDA)-approved medications contain pharmacogenomic information within the drug labeling.3 Advances in genetic sequencing techniques since the completion of the Human Genome Project have resulted in significant improvements in test efficiency, cost reduction, availability of commercial pharmacogenetic testing kits, and growth of specialized pharmacogenomics laboratories.4,5 Large-scale research efforts, such as the National Institutes of Health (NIH)-funded All of Us Research Program are advancing the discovery of the roles of the environment, lifestyle, and the genome on medication response and disease treatment in previously underrepresented populations.6 Robust resources to guide clinicians with incorporating pharmacogenetic information to guide pharmacotherapy decisions now exist for numerous commonly used medications and drug classes.7,8
These advances in research, technology, and clinical resources have now made possible the vision of incorporating genetic-level data into everyday clinical care on a wider scale, with pharmacists having a critical leadership role in guiding the rational use of pharmacogenetic testing in delivering value-based care.9 This article will provide an overview of germline pharmacogenetic targets of interests (Table 1) and the clinical and education resources available to pharmacists to advance their understanding and utilization of pharmacogenomics into clinical care.
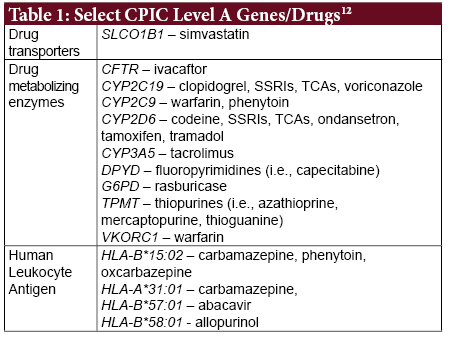
Genes of Interest
Drug Transporters
Influx and efflux transporters facilitate drug transport between the intracellular and extracellular space and play important roles in the pharmacokinetics of numerous medications. Genetic variations in genes coding for drug transporter proteins can impact transporter function and ultimately intra- and extracellular drug concentration that affect drug absorption, distribution, and elimination at the intended site.10
Drug Metabolizing Enzymes
Genetic variations in numerous drug metabolizing enzymes are a source of interindividual differences in phase I and phase II metabolism.10 Changes in drug metabolizing enzyme activity can potentially impact not only parent drug concentration, but also active or toxic metabolite concentration. Numerous genes of interest for which pharmacogenetic testing is available are of clinical consequence to cardiovascular, psychiatric, pain, and antineoplastic medications.
Human Leukocyte Antigen (HLA)
HLA is an essential protein in the immune response responsible for foreign antigen presentation to white blood cells. Several alleles in the HLA-A and HLA-B classes are associated with an increased risk of potentially fatal dermatologic hypersensitivity reactions such as Stevens-Johnson Syndrome and toxic epidermal necrolysis, which can result from the use of antiepileptic and antiretroviral medications.11
Selected Guideline & Implementation Resources
Clinical Pharmacogenetics Implementation Consortium (CPIC)
CPIC is an international consortium which develops evidence-based and peer-reviewed clinical practice guidelines to support the clinical implementation of pharmacogenetic testing and to help address the controversy surrounding the evidence level necessary to support wider pharmacogenetics use in clinical care.13 CPIC guidelines are endorsed by the American Society of Health-System Pharmacists (ASHP) and the American Society for Clinical Pharmacology and Therapeutics.14 To date, over 30 guidelines are available for numerous gene-drug pairs and are publicly accessible online.15 CPIC guidelines are user-friendly and are useful for translating pharmacogenetic test results into actionable prescribing decisions. CPIC guidelines, however, are not developed to specifically address when pharmacogenetic testing should be ordered.
IGNITE SPARK Toolbox
The SPARK toolbox is a publicly accessible online repository of patient, provider, and learner education materials; clinical implementation resources; research and data collection tools; and test reimbursement and payment resources developed by member institutions of the NIH-funded IGNITE Network.16
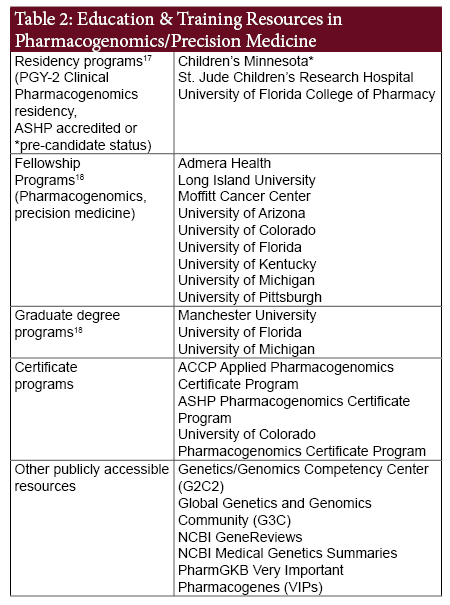
Education & Training Resources
In addition to post-graduate residency and fellowship training programs, multiple institutions have developed online pharmacogenomics education resources and offer certificate and graduate degree programs in pharmacogenomics and implementation. Several national professional organizations offer live and online certificate programs for members and non-members. Additionally, continuing education sessions focusing on pharmacogenomics knowledge, implementation, and clinical applications are commonly offered at major professional meetings. Table 2 lists various education and training resources in pharmacogenomics and precision medic
The Pharmacist’s Role
Despite advances in technology, cost, evidence, and clinical resources, barriers related to variable test reimbursement, electronic health record integration, and clinician knowledge have limited the widespread adoption of pharmacogenomic testing.19-21 Implementing pharmacogenomics will require sustained institutional and multidisciplinary commitment for promoting pharmacogenomic testing as a strategy to further enhancing patient and medication use safety.
The ASHP Statement on the Pharmacist’s Role in Clinical Pharmacogenomics has called upon pharmacists to assume leadership roles in pharmacogenomics implementation, practice, education, and research.22 The value of the pharmacist’s role in pharmacogenomics practice has also been recognized by genetics disciplines.23 Pharmacists must take an active role in educating and guiding health care providers with pharmacogenetic testing recommendations, result interpretation, and application of test results to medication selection and use. Indeed, pharmacists are already fulfilling these implementation, educational, and clinical roles through the establishment and leadership of inpatient clinical pharmacogenomics services, pharmacogenomics specialty clinics, and molecular tumor boards.24-26 Pharmacists will also play a critical role in educating patients and providers on the use, benefits, risks, and limitations of direct-to-consumer genetic testing as its ubiquity and use increases.27-29
Conclusion
Pharmacists are well placed to promote and lead pharmacogenomics implementation, utilization, and patient and provider education given their accessibility and position as medication use experts. Numerous educational, clinical, and implementation resources and guidelines are available to support pharmacists as they promote and lead pharmacogenomics implementation, practice, and research efforts.