Print This Article
Best Practice Journal Article
Reduction of Harm from Opioid Therapy Through Focused Improvements in the Opioid Medication Use Process
Feature Article
by Adam Bursua, PharmD, BCPS, Patient Safety Operational Lead, UI Health - Chicago, IL; Co-authors: Connie Larson, PharmD, Senior Associate Director, Safety and Quality, UI Health - Chicago, IL; Jane McCullough, PharmD, PGY-1 Pharmacy PRactice Resident, UI Health - Chicago, IL
Introduction, Purpose, and Goals of the Program
Opioid analgesics are widely used for the management of pain. These medications are associated with a number of serious side effects, including respiratory depression and sedation, which could ultimately lead to death.1-3 Increased prescribing and utilization of opioids has led to an epidemic of opioid related harm in United States. Among the 70,200 drug overdose deaths in 2017, approximately 68% were attributed to opioids, including prescription opioids.1,2 The Centers for Disease Control and Prevention reports that over 130 individuals die each day from an opioid-related overdose. In 2017, more than 191 million opioids were prescribed to patients across the United States and approximately 1.4 million people misuse prescription opioids.3 In the inpatient setting, adverse effects from opioids prescribed to patients during their hospital stay have contributed to increased length of hospitalization, increased healthcare costs, and unexpected deaths.4,5 As the opioid epidemic continues to evolve, interventions throughout the medication use process must be considered in order to change the trajectory of the crisis while protecting patient access to safe opioid analgesic therapy.
In an effort to eliminate preventable patient harm, the Medication System Review Committee (MSRC) at our institution was asked to lead a medication harm reduction effort as part of a Zero Harm Initiative (ZHI) program at our health system. The Medication Safety ZHI is focused on reduction of preventable adverse events related to the use of opioid analgesics. In order to assess our safe opioid use and identify opportunities to improve the opioid medication use process, a naloxone root cause analysis trigger tool was implemented in January of 2017. Naloxone is an antidote indicated for the reversal of opioid-induced respiratory depression and sedation. Naloxone is often used in the inpatient setting if a patient experiences serious adverse effects related to opioid therapy.6,7 The Agency for Healthcare Research and Quality (AHRQ), the Institute for Safe Medication Practices (ISMP), and the Hospital Improvement Innovation Network (HIIN) recommends such a strategy of using clinical “trigger tools” to identify medication errors and adverse drug events.6-8 This process is more effective than relying upon voluntary reporting alone because it detects more cases of potential patient harm and allows for a retrospective review of these cases in more detail.
The overarching program objective is to address the opioid public health crisis and reduce harm from opioid therapy through pharmacy-led, multidisciplinary medication safety interventions. The goals of the program are to investigate harm events to inform potential improvements, identify risk trends associated with iatrogenic opioid overdose, and to implement system-level interventions to modify opioid prescribing behaviors and improve monitoring practices. Other goals of the program include:
- Development of a standardized and accurate metric for measurement of opioid-related harm
- Increasing access to opioid utilization and harm data for stakeholders charged with improving the opioid medication use process
- Maintaining a naloxone data registry to monitor trends in opioid related harm and to measure the effectiveness of interventions over time
Description of the Program
In the 4th Quarter of 2016, planning for the Medication Safety Opioid ZHI began with establishing outcome and process metrics for measuring performance and progress. Referencing AHRQ, ISMP, and IHI, the committee concluded that the main outcome metric would be to utilize the naloxone trigger tool.6-8 This metric served two purposes. First, it served as a consistent measure of iatrogenic opioid related harm. Second, it enabled performance of a standardized root cause analysis to be performed, with the goal of identifying areas for intervention and improvement. To allow for this metric to be as sensitive and specific as possible for measurement of the intended outcome, naloxone dispensing was standardized to automated dispensing cabinets. Once this dispensing process was updated, a standardized review process for each naloxone dispensation was initiated. Other metrics were also established, and they included tracking of opioid doses dispensed, utilization of opioids for mild pain indications, compliance with opioid monitoring recommendations, and measures of opioid related harm normalized to the number of patients at risk.
As described above, in January of 2017, our institution implemented a naloxone root cause analysis tool to identify patients that experienced adverse effects as a result of the administration of opioids during their admission. Each month, data from each naloxone dispensation has been collected via retrospective chart review (Figure 1). In addition, opioid utilization data is collected and analyzed. The data regarding opioid administration is stored on a Microsoft Excel database and contains information regarding the opioid administered, route, frequency, medical service, and date of administration. Exclusion criteria is applied to remove naloxone use not associated with iatrogenic opioid use.
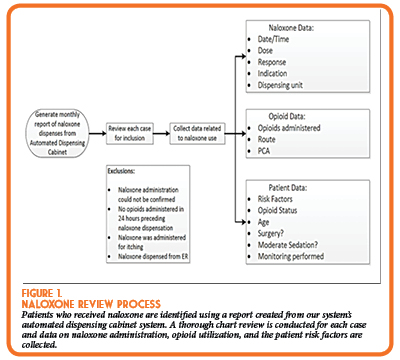
The data has been used to identify high risk opioid use and performance deficiencies associated with iatrogenic opioid overdose. Some areas which were identified as contributing to iatrogenic opioid overdose within our institution included morphine administered to patients with renal dysfunction (defined as a creatinine clearance less than 50 mL/min), opioid use for mild pain indications, and inconsistent performance of sedation assessments prior to opioid administration. In response to these findings, system level interventions were planned and implemented.
Several modifications to prescribing behaviors were made in the medication use process including:
- An alert was implemented into the institution's computerized prescriber order entry (CPOE) system to caution prescribers against ordering morphine for patients with reduced renal function, as the active metabolite of morphine can accumulate in those with renal dysfunction and pose a serious risk of developing respiratory depression and/or sedation.
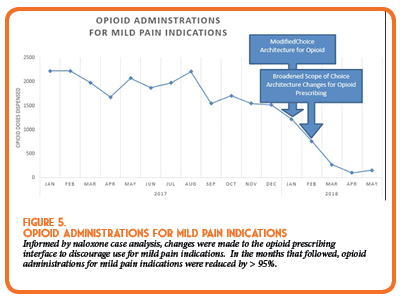
- The “mild pain” indication was removed as an available ordering option in the CPOE system to nudge prescribers to avoid opioids for less severe pain and to use non-opioid alternatives instead.
- Order sets were modified to reduce reliance on opioid therapies and encourage utilization of multimodal analgesia within the institution.
Similarly, system improvements were made to improve monitoring of patients receiving opioids after opportunities were identified. These improvements include:
- Requirement for end-tidal carbon dioxide monitoring for patients receiving PCA therapy.
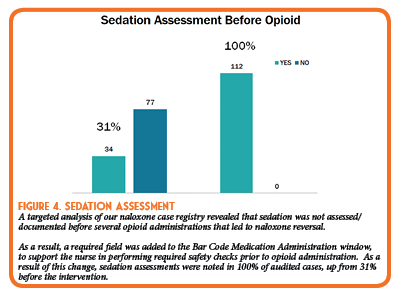
- Requirement for documentation of sedation assessment was implemented via barcode medication administration (BCMA) of opioid drugs.
- A report/notification was developed and configured to be sent/reviewed by our moderate sedation committee whenever a naloxone case was temporally associated with a moderate sedation procedure.
The success of these interventions are measured through continued monitoring of opioid use and iatrogenic opioid harm through the outcome and process measures outlined above. Sharing of this data with other stakeholders has enabled them to measure progress with other opioid related initiatives throughout the health system.
Experience with and Outcomes of the Program
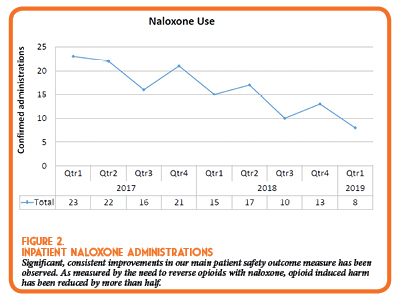
The program has documented significant improvements in several important process measures, as well as the main outcome measure, reduction of harm from opioid therapy. The number of naloxone administrations for opioid-induced respiratory depression/sedation has continued to significantly decrease from 29 administrations in Quarter 1 of 2017 to only 8 administrations in the latest reported quarter in 2019 (Figure 2). Using the Illinois Hospital Association’s calculator for cost savings from adverse drug events, the health system averted an estimated $385,000 of costs for adverse drug events related to opioid therapy.
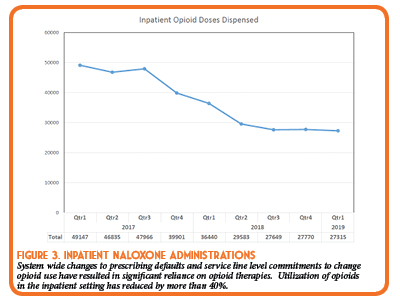
In that same time period, the amount of opioids administered has also steadily and significantly decreased. Since implementing this initiative, the number of inpatient opioid administrations went from 49,147 quarter 1 of 2017 to 27,315 quarter 1 of 2019 (Figure 3).
Several process measures have also improved as a result of the implemented opioid safety strategies. Of note, there have been significant improvements in sedation monitoring by nursing staff (Figure 4) and a reduction in opioid use for mild pain indications (Figure 5).
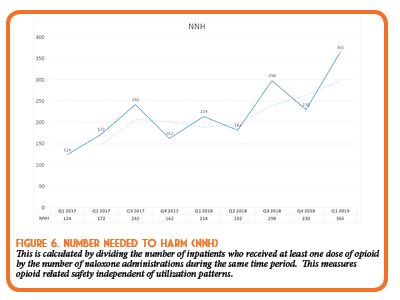
Overall, our efforts have demonstrated a reduction in both naloxone and opioid administrations within our institution. Independent of opioid utilization figures, our institution has seen a decrease in opioid related harm, as illustrated in the “Number Needed to Harm” calculation in Figure 6. In the first quarter after implementation of this program, only 124 patients receiving opioid therapy would be treated with an opioid before one patient would experience an opioid adverse event requiring naloxone administration. After the described interventions, as of Quarter 1 of 2019, the number needed to harm has increased to 365 patients who would receive opioid therapy before a naloxone administration is required. When analyzed together, these findings suggest opioid safety has improved through both conservative opioid utilization, and improved safety/monitoring when opioids are used.
Discussion of Innovative Aspects of Programs and Achievement of Goals
Since its development in 2017, the naloxone trigger tool has enabled the implementation of several innovative, system-level interventions to improve opioid prescribing behaviors and monitoring practices. While utilization of the naloxone trigger tool for both root cause analysis and safety benchmarking is in itself innovative, other innovative aspects of the program came from the improvement interventions.
Many of the interventions implemented over this time period relied on the innovative behavioral economic principle of the “Nudge”. The 2017 Nobel Prize for Economics was awarded to University of Chicago Economics Professor Richard Thaler, who described this theory of decision behavior. The theory describes methods to alter decision making behavior through modification of choice architecture, intentionally setting default options, or otherwise “nudging” individuals to make the ‘right’ decision without significantly changing incentives. The “nudge” theory was applied in several of the interventions implemented through our Zero Harm Initiative. One example is the modification of opioid prescribing for mild pain indication by modification of prescriber “choice architecture” (Figure 5). Another example was the modification of order defaults for our most commonly prescribed opioid medication, acetaminophen/hydrocodone. Each of these interventions dramatically changed opioid prescribing behavior, and each relied on the innovative theory of the “nudge”. In the case of the acetaminophen/hydrocodone “nudge”, prescribing was reduced by more than 20% for an average of approximately 20,000 fewer tablets each month after the intervention.
Conclusions
Through the combination of innovative practices for both measurement of patient related harm and process improvement science, our pharmacy-led initiative significantly improved the safety of opioid use in our health system.
References
- Scholl L, Seth P, Kariisa M, Wilson N, Baldwin G. Drug and Opioid-Involved Overdose Deaths — United States, 2013–2017. MMWR Morb Mortal Wkly Rep 2019;67:1419–1427.
- About the U.S. Opioid Epidemic. HHS. 2019. Available at: https://www.hhs.gov/opioids/about-the-epidemic/index.html.
- Centers for Disease Control and Prevention. 2018 Annual Surveillance Report of Drug-Related Risks and Outcomes — United States. Surveillance Special Report. Centers for Disease Control and Prevention, U.S. Department of Health and Human Services. Published August 31, 2018. Accessed June 5, 2019 from https://www.cdc.gov/drugoverdose/pdf/pubs/2018- cdc-drug-surveillance-report.pdf.
- Lynn LA, Curry JP. Patterns of unexpected in-hospital deaths: a root cause analysis. Patient Saf Surg. 2011;5:3.
- Oderda GM, Said Q, Evans RS, et al. Opioid-related adverse drug events in surgical hospitalizations: impact on costs and length of stay. Annals of Pharmacotherapy. 2007; 41(3): 400–407.
- Agency for Healthcare Research and Quality. Triggers and trigger tools. United States Department of Health and Human Services Patient Safety Network. https://psnet.ahrq.gov/. June 2017. Accessed June 5, 2019.
- Measuring up to medication safety. Institute for Safe Medication Practices. https://www.ismp.org/resources/measuring-medication-safety. March 10, 2005. Accessed June 5, 2019.
- Health Research & Education Trust. Adverse Drug Events Change Package: 2017 Update. 2017. Chicago. IL: Health Research and Education Trust. Accessed at www.hret-hiin.org. Accessed June 5, 2019.

Once you're logged in to CESally.com, type "2019 ICHP Best Practice on CEsally.com" and you will be able to chose the Pharmacist or the Pharmacy Technician box.